Piperacillin sodium-tazobactam sodium medicinal composition microsphere injection
A technology of tazobactam sodium and piperacillin sodium, which is applied in the field of medicine and can solve problems such as large burst release, difficulty in large-scale preparation, and poor process reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
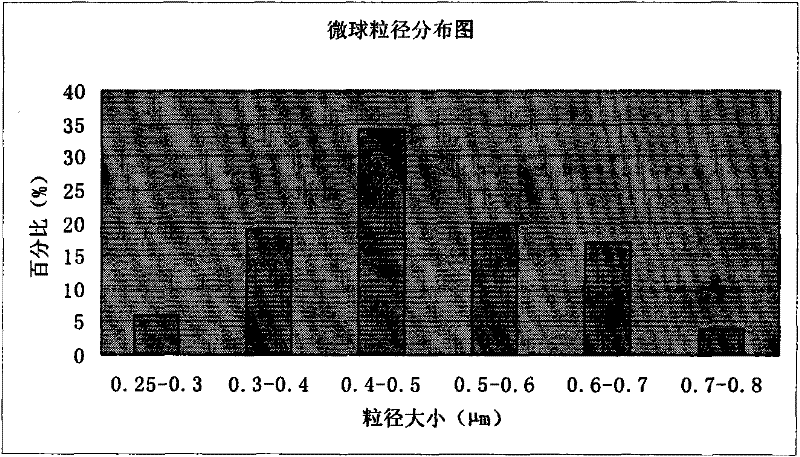
[0073] Example 1 Preparation of microsphere injection of pharmaceutical composition of piperacillin sodium and tazobactam sodium
[0074] Prescription: (100 bottles)
[0075] Piperacillin Sodium 40g
[0076] Tazobactam Sodium 10g
[0077] PLA-PEG 40g
[0078] PEG600 60g
[0079] Polysorbate 80 10g
[0080] Mannitol 20g
[0081] Preparation Process:
[0082] (1) Dissolve 40g piperacillin sodium, 10g tazobactam sodium, 60gPEG600 and 20g mannitol in 800ml water for injection to obtain an aqueous phase;
[0083] (2) Dissolve 40g PLA-PEG and 10g polysorbate 80 in 200ml dichloromethane to obtain an oil phase;
[0084] (3) Slowly drip the water phase obtained above into the oil phase under stirring conditions, stir for 10 minutes after dripping, and then transfer to a high-speed homogenizer and stir at high speed for 3 times, each time for 10 minutes, to obtain a uniform white emulsion ;
[0085] (4) Put the white emulsion into the spray dryer, adjust the spray conditions (inlet temperature 65°C, ou...
Embodiment 2
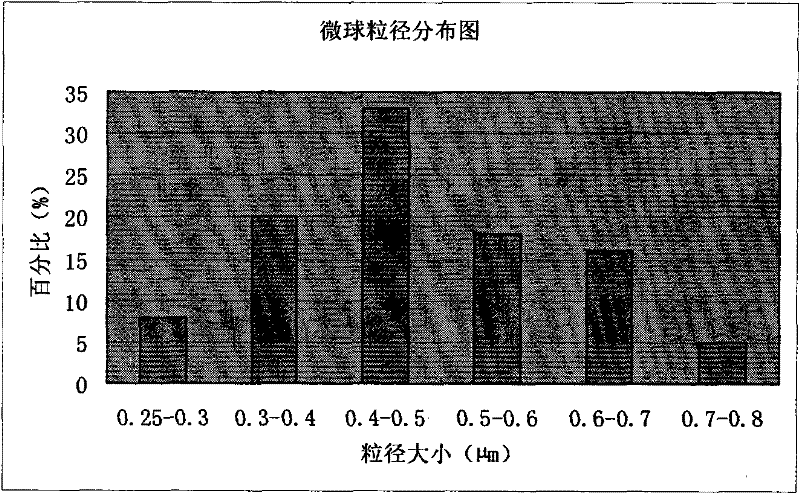
[0101] Example 2 Preparation of microsphere injection of pharmaceutical composition of piperacillin sodium and tazobactam sodium
[0102] Prescription: (100 bottles)
[0103] Piperacillin Sodium 80g
[0104] Tazobactam Sodium 10g
[0105] PLA-PEG 55g
[0106] PEG600 70g
[0107] Polysorbate 80 15g
[0108] Mannitol 20g
[0109] Preparation Process:
[0110] (1) Dissolve 80g piperacillin sodium, 10g tazobactam sodium, 70gPEG600 and 20g mannitol in 1000ml water for injection to obtain an aqueous phase;
[0111] (2) Dissolve 55g PLA-PEG and 15g Polysorbate 80 in 300ml of toluene to obtain an oil phase;
[0112] (3) Slowly drip the water phase obtained above into the oil phase under stirring conditions, stir for 10 minutes after dripping, and then transfer to a high-speed homogenizer and stir at high speed for 3 times, each time for 10 minutes, to obtain a uniform white emulsion ;
[0113] (4) Put the white emulsion into the spray dryer, adjust the spray conditions (inlet temperature 70°C, outlet...
Embodiment 3
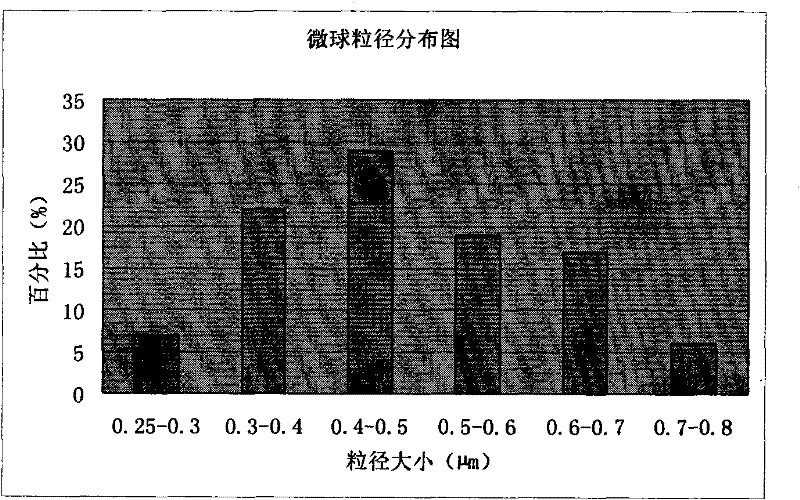
[0129] Example 3 Preparation of microsphere injection of pharmaceutical composition of piperacillin sodium and tazobactam sodium
[0130] Prescription: (100 bottles)
[0131] Piperacillin Sodium 80g
[0132] Tazobactam Sodium 10g
[0133] PLA-PEG 60g
[0134] PEG600 65g
[0135] Polysorbate 80 22g
[0136] Mannitol 30g
[0137] Preparation Process:
[0138] (1) Dissolve 80g piperacillin sodium, 10g tazobactam sodium, 65gPEG600 and 30g mannitol in 1200ml water for injection to obtain an aqueous phase;
[0139] (2) Dissolve 60g PLA-PEG and 22g polysorbate 80 in 400ml acetone to obtain an oil phase;
[0140] (3) Slowly drip the water phase obtained above into the oil phase under stirring conditions, stir for 10 minutes after dripping, and then transfer to a high-speed homogenizer and stir at high speed for 5 times, each time for 10 minutes, to obtain a uniform white emulsion ;
[0141] (4) Put the white emulsion into the spray dryer, adjust the spray conditions (inlet temperature 75°C, outlet te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com