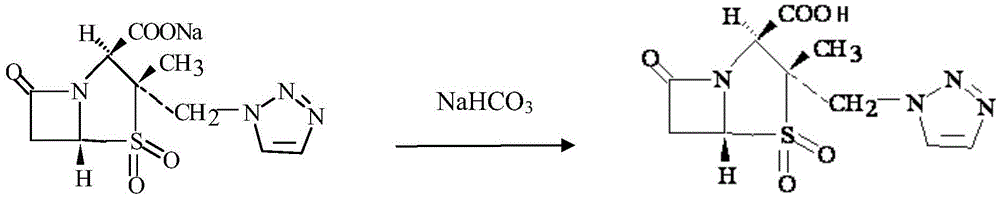
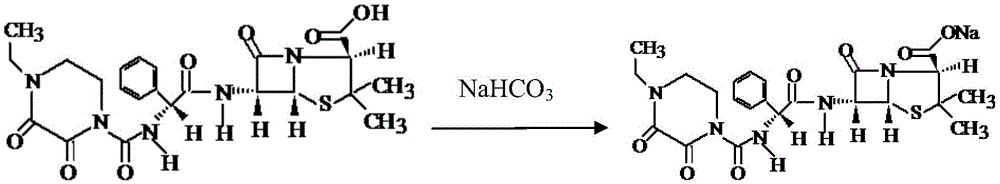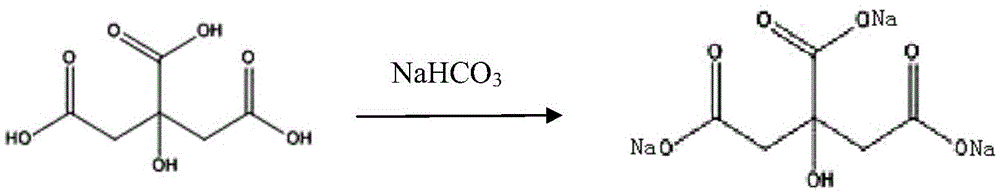Preparation method of piperacillin sodium and tazobactam sodium for injection
A technology for piperacillin sodium and tazobactam sodium, which is applied in the field of medicine, can solve the problems of lowering and lowering the content of piperacillin sodium and tazobactam sodium, and achieves improved acidity stability, improved storage stability, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 102ml of bottom water into the batching tank, cool down to 6.5°C; put 4.28g of citric acid into the batching tank, stir for 10 minutes, then slowly add 5.14g of sodium bicarbonate (control the time of adding alkali for not less than 20 minutes) ), record the pH after 10 minutes, if the pH is lower than 6.3, add an appropriate amount of sodium bicarbonate to adjust; if the pH value is higher than 6.6, add an appropriate amount of citric acid to adjust, and finally adjust the pH to 6.40;
[0028] (2) temperature control 5~10 ℃, 125g piperacillin acid is transferred in the batching tank, then the sodium bicarbonate solution (19.40g sodium bicarbonate is dissolved in water to be made into 14% solution) with concentration is 14% dropwise Add it to the batching tank, and control the pH during the dropping process not to be higher than 7.0;
[0029] (3) continue to add tazobactam 14.8g, then the 14% sodium bicarbonate solution prepared (4.13g sodium bicarbonate is diss...
Embodiment 2
[0033] (1) 115ml of bottom water was cooled to 6.0°C, and 4.28g of citric acid and 5.14g of sodium bicarbonate were added. Adjust pH6.36;
[0034] (2) Add 125 g of piperacillin, 14.8 g of tazobactam, and dropwise add sodium bicarbonate: a total of 23.53 g (first 19.40 g and then 4.13 g, prepared into a solution with a concentration of 15%). Detect the final pH6.25;
[0035] (3) At this time, the concentration of the feed solution was 38%. The feed solution was sterilized, filtered, and freeze-dried to obtain the original powder of piperacillin sodium and tazobactam sodium (8:1).
[0036] All the other are with embodiment 1.
Embodiment 3
[0038] (1) 93ml of bottom water, lower the temperature to 7.0°C, add 4.28g of citric acid and 5.14g of sodium bicarbonate, and adjust the pH to 6.37;
[0039] (2) Add 125 g of piperacillin, 14.8 g of tazobactam, and add dropwise a total of 23.53 g of sodium bicarbonate (19.40 g first and 4.13 g later, to prepare a solution with a concentration of 13%). Detect the final pH6.24;
[0040] (3) At this time, the concentration of the feed solution was 38%. The feed solution was sterilized, filtered, and freeze-dried to obtain the original powder of piperacillin sodium and tazobactam sodium (8:1).
[0041] All the other are with embodiment 1.
[0042] The performance index of the product of the embodiment of the present invention 1-3 and the product of comparative example (step (1) only adds bottom water, promptly does not add citric acid and sodium bicarbonate, all the other are the same as embodiment 1) as shown in table 1.
[0043] The product stability data (24 months) of table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com