Piperacillin sodium and tazobactam sodium pharmaceutical composition and preparation method thereof
A technology of tazobactam sodium and piperacillin sodium, which is applied in the field of medicine, can solve the problems of poor stability of piperacillin sodium content, no crystallization and degradation products, etc., and achieve satisfactory clarity, rapid drying, and easy control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
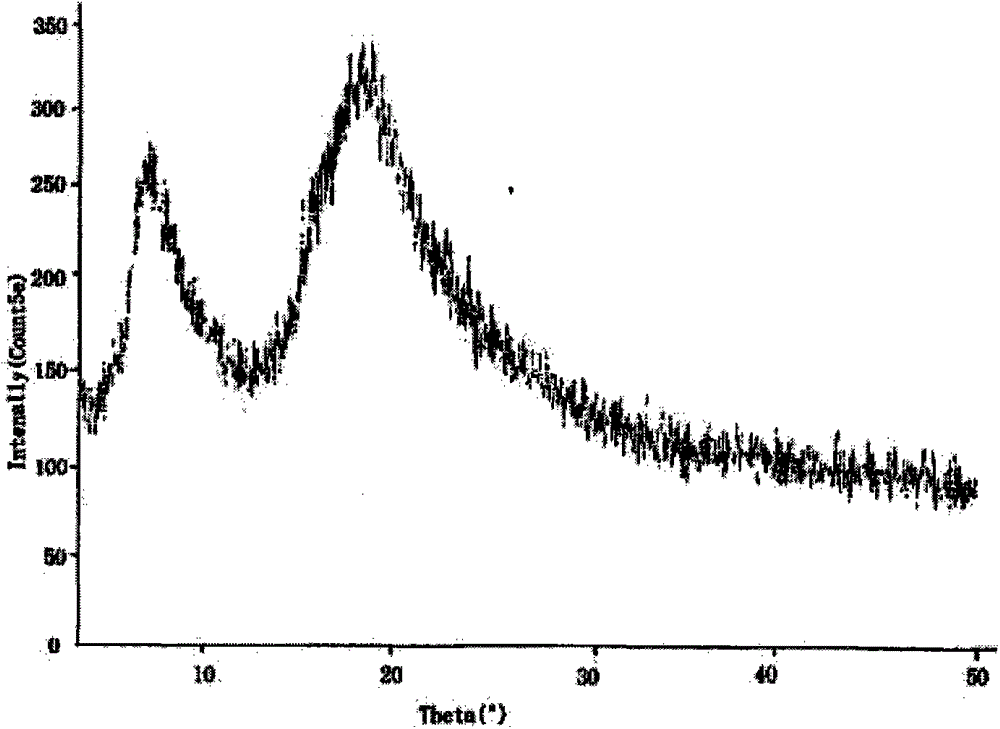
Image
Examples
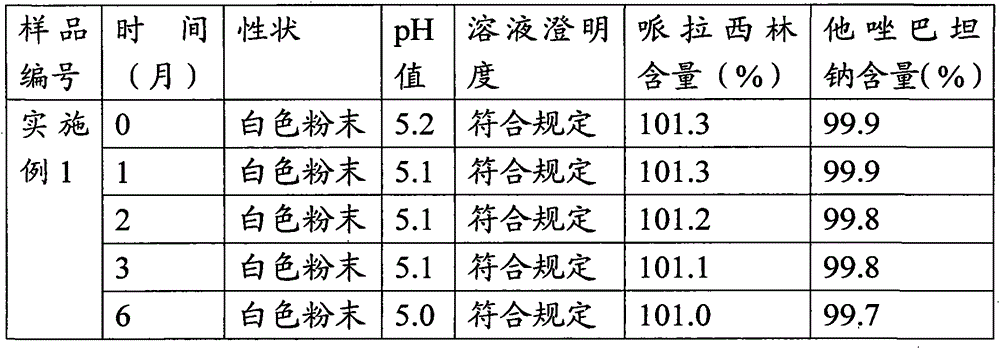
Embodiment 1
[0023] A pharmaceutical composition of piperacillin sodium and tazobactam sodium, comprising piperacillin sodium and tazobactam sodium, and also comprising disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair, and its specific preparation steps are as follows:
[0024] (A) accurately weigh 100g piperacillin sodium and 25g tazobactam sodium, join in 1000ml of water for injection, fully stir;
[0025] (B) Add disodium hydrogen phosphate-sodium dihydrogen phosphate buffered solution with a concentration of 0.2M and a pH of 5.8 dropwise under stirring until the pH value is adjusted to 5.0, continue stirring for 30 minutes after the addition is completed, and sterilize and filter;
[0026] (C) The solution obtained in (B) was spray-dried, aseptically pulverized, and aseptically distributed to obtain 100 bottles of piperacillin sodium-tazobactam sodium powder injection preparation.
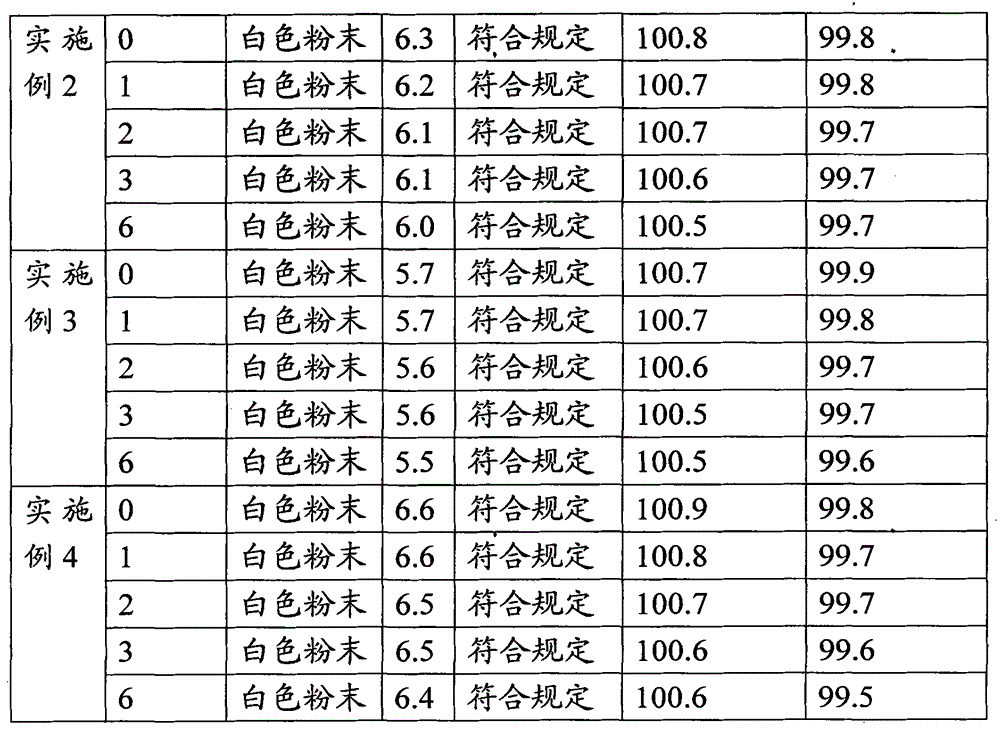
Embodiment 2
[0028] A pharmaceutical composition of piperacillin sodium and tazobactam sodium, comprising piperacillin sodium and tazobactam sodium, and also comprising disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair, and its specific preparation steps are as follows:
[0029] (A) accurately weigh 100g piperacillin sodium and 25g tazobactam sodium, join in 1000ml of water for injection, fully stir;
[0030] (B) Add dropwise a disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution with a concentration of 0.2M and a pH of 6.5 under stirring until the pH value is adjusted to 6.0. After the addition is completed, continue to stir for 30 minutes, and then sterilize and filter;
[0031] (C) The solution obtained in (B) was spray-dried, aseptically pulverized, and aseptically distributed to obtain 100 bottles of piperacillin sodium-tazobactam sodium powder injection preparation.
Embodiment 3
[0033] A pharmaceutical composition of piperacillin sodium and tazobactam sodium, comprising piperacillin sodium and tazobactam sodium, and also comprising disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pair, and its specific preparation steps are as follows:
[0034] (A) accurately weigh 100g piperacillin sodium and 25g tazobactam sodium, join in 1000ml of water for injection, fully stir;
[0035] (B) Add disodium hydrogen phosphate-sodium dihydrogen phosphate buffered solution with a concentration of 0.2M and a pH of 6.0 dropwise under stirring until the pH value is adjusted to 5.5, continue stirring for 30 minutes after the addition is completed, and sterilize and filter;
[0036] (C) The solution obtained in (B) was spray-dried, aseptically pulverized, and aseptically distributed to obtain 100 bottles of piperacillin sodium-tazobactam sodium powder injection preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com