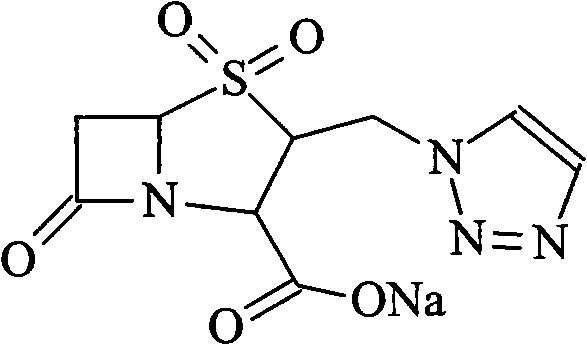
High-purity tazobactam sodium compound
A technology for tazobactam sodium and compound, which is applied in the refining field of tazobactam sodium compound, can solve the problems of affecting clinical application, difficult reaction, decreased preparation stability, etc., and is suitable for large-scale industrial production and improves clinical application. The effect of medication, the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Purification of Tazobactam Sodium
[0031] (1) Dissolve 100g of tazobactam sodium crude product in 1500ml of water, add 0.01mol / L sulfuric acid solution to adjust the pH value to 2.0, stir to precipitate insoluble matter, filter, and obtain a solid after washing with purified water;
[0032] (2) the solid obtained in step (1) is dissolved in 1000ml of a mixture of dichloromethane and methanol (volume ratio 3: 2), added to a column filled with D101 macroporous resin and passed through, and then mixed with dichloromethane and ethyl acetate (volume Ratio 1: 2) 500ml of the mixture was eluted and purified as the eluent, and the eluate was collected;
[0033] (3) Adjust the pH value of the eluate obtained in step (2) to 5.5 with 5% sodium isooctanoate solution, and precipitate a solid, centrifuge for 10 min, wash with 500 ml of ethyl acetate, and vacuum-dry at 50° C. for 6 hours to obtain a refined product 93.1g, yield 93.1%, HPLC detection purity 99.95%.
Embodiment 2
[0035] Purification of Tazobactam Sodium
[0036] (1) Dissolve 100g of tazobactam sodium crude product in 1500ml of water, add 0.05mol / L hydrochloric acid solution to adjust the pH value to 3.0, stir to precipitate insoluble matter, filter, and obtain a solid after washing with purified water;
[0037] (2) Dissolve the solid obtained in step (1) with 1000ml of a mixture of dichloromethane and methanol (volume ratio 3:2), add to the column filled with AB-8 macroporous adsorption resin and pass through, then dichloromethane and ethyl acetate Esters (volume ratio 1: 2) mixture 500ml are used as eluent for elution and purification, and the eluate is collected;
[0038] (3) The eluent obtained in step (2) was adjusted to a pH value of 6.5 with 5% sodium bicarbonate solution, and a solid was precipitated, centrifuged for 20 min, washed with 500 ml of ethyl acetate, and vacuum-dried at 60° C. for 4 hours to obtain a refined product 92.2g, yield 92.2%, HPLC detection purity 99.92%. ...
Embodiment 3
[0039] The refining of embodiment 3 tazobactam sodium
[0040] (1) Dissolve 100g of tazobactam sodium crude product in 1500ml of water, add 0.1mol / L phosphoric acid solution to adjust the pH value to 4.0, stir to precipitate insoluble matter, filter, and obtain a solid after washing with purified water;
[0041] (2) Dissolve the solid obtained in step (1) with 1000ml of a mixture of dichloromethane and methanol (volume ratio 3:2), add to the column filled with AB-8 macroporous adsorption resin and pass through, then dichloromethane and ethyl acetate Esters (volume ratio 1: 2) mixture 600ml is eluted and purified as an eluent, and the eluate is collected;
[0042] (3) Adjust the pH value of the eluate obtained in step (2) to 8.0 with 0.01mol / L sodium acetate solution, and precipitate a solid, centrifuge for 10 min, wash with 500 ml of ethyl acetate, and vacuum-dry at 55°C for 5 hours to obtain the essence The product is 91.7g, the yield is 91.7%, and the purity detected by HPLC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com