Liposome injection prepared from ceftriaxone sodium tazobactam sodium medicinal composition
A technology of tazobactam sodium and ceftriaxone sodium, which is applied in the direction of liposome delivery, active ingredients of heterocyclic compounds, antibacterial drugs, etc., can solve problems such as the limitation of preparation technology means, achieve simple and effective preparation process, improve Curative effect, effect of prolonging action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of ceftriaxone sodium tazobactam sodium drug mixture liposome
[0068] Prescription (100 bottles):
[0069] Ceftriaxone Sodium 75g
[0070] Tazobactam Sodium 25g
[0071] Dimyristoylphosphatidylcholine 66.7g
[0072] Soy Phosphatidylinositol 33.3g
[0073] Phosphatidylserine 32.5g
[0074] Octadecylamine 32.5g
[0075] Mannitol 50g
[0076] Trehalose 100g
[0077] Sodium metabisulfite 3.33g
[0078] Vitamin C 1.67g
[0079] making process:
[0080] (1) Dissolving 66.7g of dimyristoylphosphatidylcholine, 33.3g of soybean phosphatidylinositol, 32.5g of phosphatidylserine, and 32.5g of octadecylamine in 1200ml of isopropanol and tris In the mixed solvent of methyl chloride, the organic solvent was removed under reduced pressure on a rotary film evaporator to obtain a phospholipid film;
[0081] (2) Add 600ml of sodium dihydrogen phosphate-disodium hydrogen ph...
Embodiment
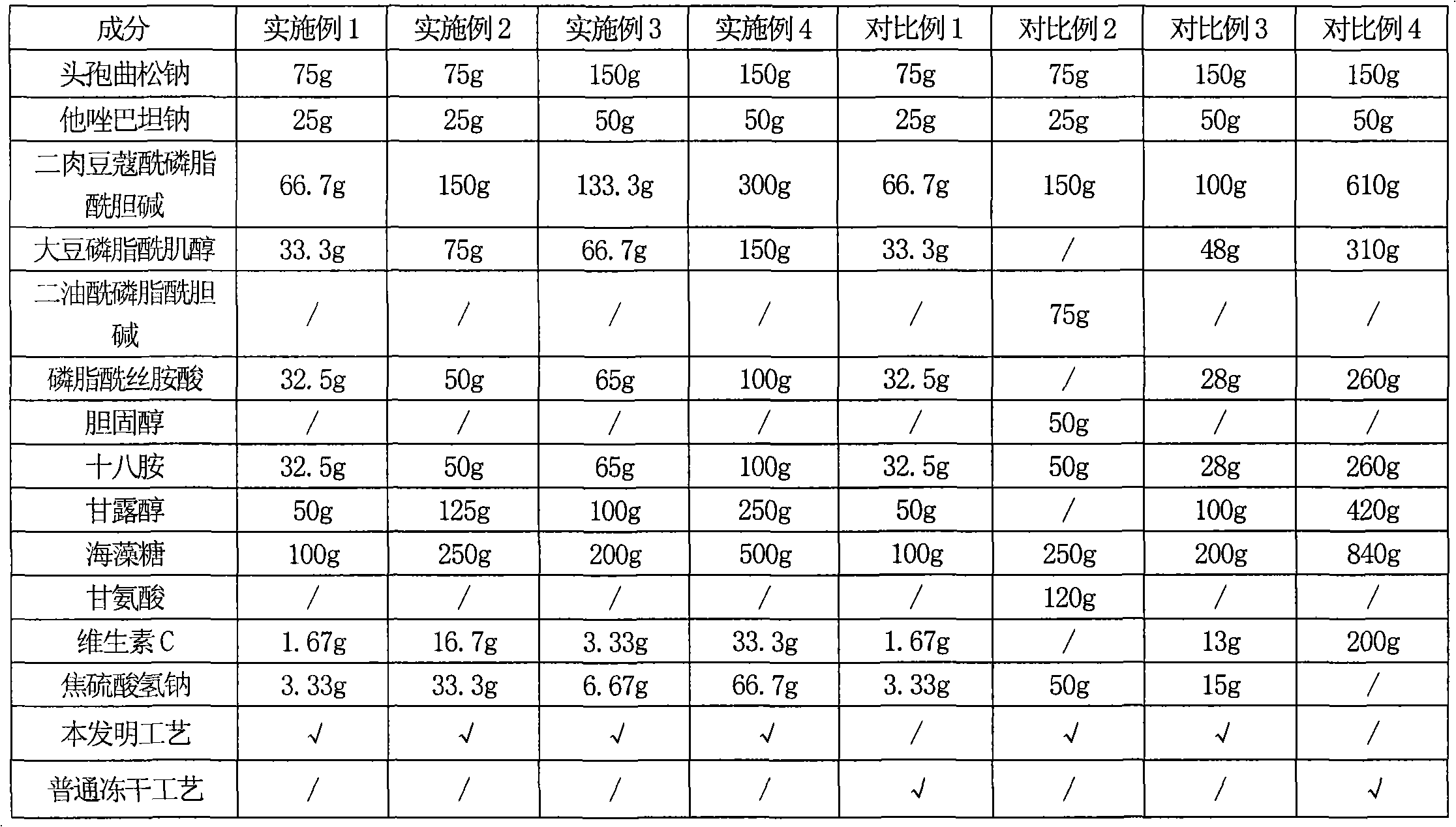
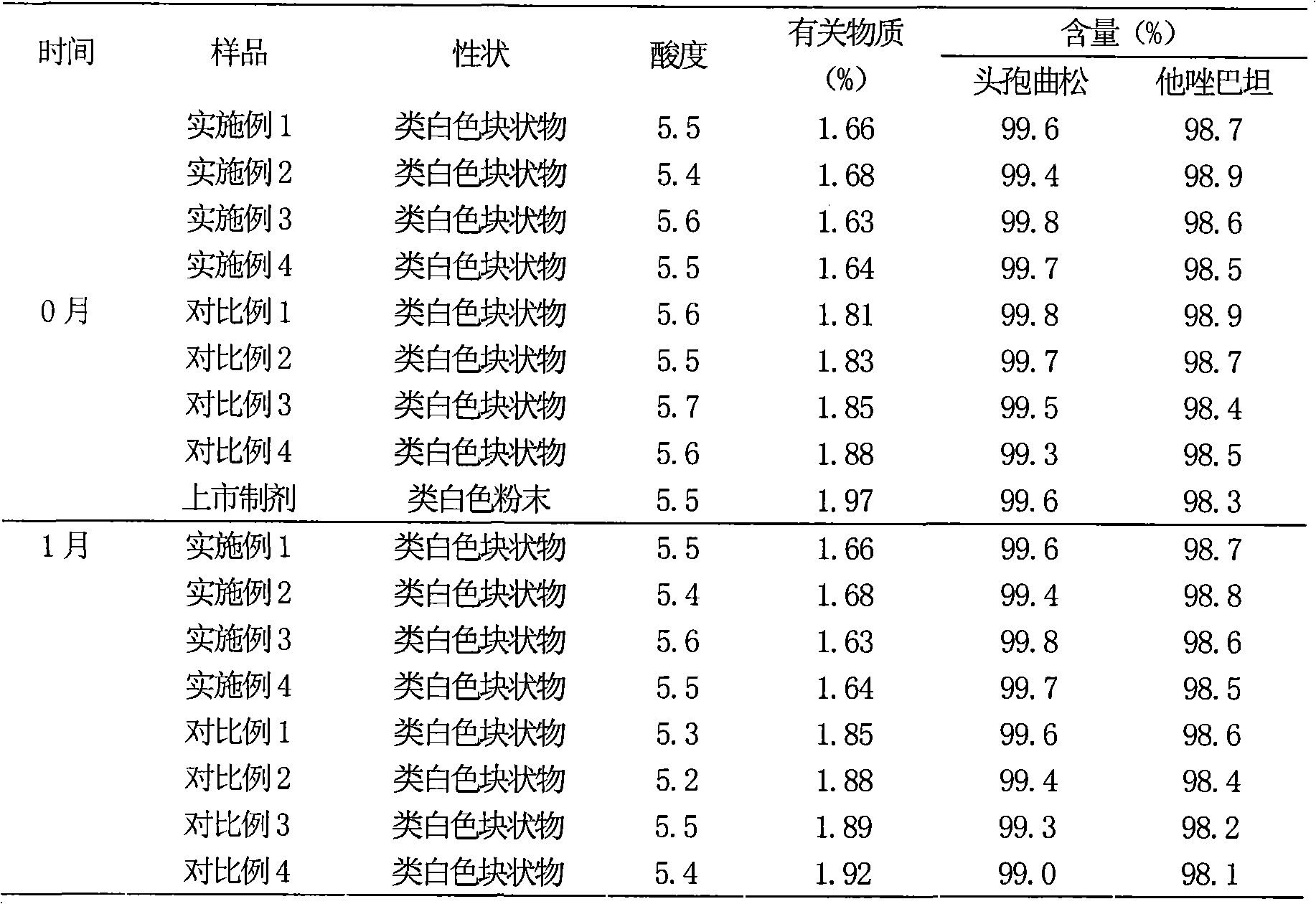
[0104] Embodiment and comparative example comparison Comparison of the preferred auxiliary material proportioning and freeze-drying process of the present invention
[0105] In the following table, the following examples and comparative examples are designed as experimental factors with the selected auxiliary materials and consumption and preparation technology of the present invention, wherein Examples 1-4 are carried out by the preparation technology of the present invention, and the auxiliary materials and consumption used are in the present invention Within the preferred range of comparative examples 1-4, the adjuvant or the amount of adjuvant used is outside the preferred range of the present invention, or some adopt the common freeze-drying process to carry out, and the results of investigation in the test example are compared and illustrated to the advantages of the present invention.
[0106] The comparison of table 1 embodiment and comparative example
[0107]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com