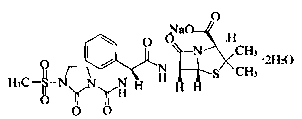
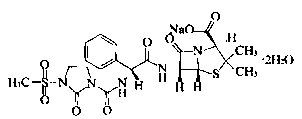
Mezlocillin sodium compound and medicine composition thereof
A technology of mezlocillin sodium and mezlocillin acid, which is applied in the field of medicine and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
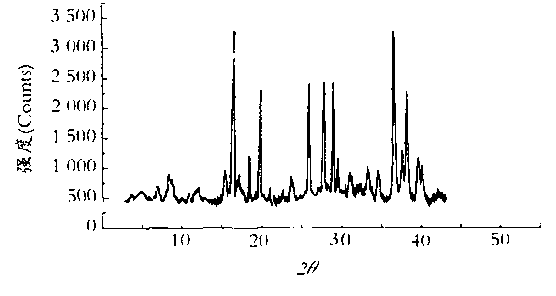
Image
Examples
Embodiment 1
[0060] 1. At 15-18°C, add 100L of a mixture of water and isopropanol (volume ratio 25:1) into the reaction kettle.
[0061] 2. Add 8Kg of ampicillin to the reaction kettle, add 1mol / L sodium bicarbonate solution dropwise while stirring, and stop the dropwise addition after the solution dissolves.
[0062] 3. Add 5Kg of 1-chloroformyl-3-methanesulfonyl-2-imidazolidinone while stirring for condensation reaction, add 1mol / L sodium bicarbonate dropwise during the reaction to control the pH value to 7.9, and keep the temperature at 15 ℃, the stirring speed is 250 rpm, and the reaction is continued for 45 minutes after the addition is completed, then the solution is filtered, and the filtrate is transferred to a crystallization tank.
[0063] 4. Add 40L of a mixture of ethanol and isopropanol (volume ratio 10:1) to the crystallization kettle, stir to mix it evenly with the feed liquid, keep the temperature at 20°C, and adjust with dilute hydrochloric acid (9.5~10.5% by weight) pH t...
Embodiment 2
[0065] 1. Add 10Kg of mezlocillin acid to 40L of water for injection, stir evenly, and cool down to 8°C.
[0066] 2. Add dropwise the mixed aqueous solution of sodium bicarbonate and sodium hydroxide (sodium bicarbonate 10%w / w, sodium hydroxide 2%w / w), adjust the pH value to 5.5, stir the reaction until the solution is completely clear and continue to stir for 30 minutes , add 5% mezlocillin acid by weight activated carbon, stir for 20 minutes, filter to remove carbon, and filter the filtrate through a 0.22 μm filter membrane to sterilize.
[0067] 3. Put the filtrate in a freeze-drying tray with a thickness of 12mm, pre-freeze at -35°C for 3 hours, then continue to cool down to -45°C, keep it for 1 hour, and then start vacuum drying. The first stage: adjust the vacuum to 35Pa , slowly warm up to -10°C (heating time 6 hours), keep at -10°C for 1 hour; second stage: slowly heat up to 0°C (≤0.1°C / min), keep at 0°C for 1 hour; third stage : 12 hours, the temperature was raised t...
Embodiment 3
[0072] Prescription: (Specification: 0.5g)
[0073] Mezlocillin sodium compound 500g (calculated as mezlocillin)
[0074] Make 1000 pieces
[0075] Process:
[0076] 1. Aseptically pass the mezlocillin sodium compound through an 80-mesh sieve under 100-grade conditions in a sterile room, and weigh the prescription amount for use.
[0077] 2. Calculate the content and fill it according to the specification to get the finished product.
[0078] The preparation of embodiment 4 injection mezlocillin sodium
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com