A kind of cefoperazone sodium tazobactam sodium pharmaceutical composition
A technology of tazobactam sodium and cefoperazone sodium, which is applied in the field of medicine, can solve the problems of poor stability, high content of related substances, uncontrollable quality and other problems of cefoperazone sodium and tazobactam sodium pharmaceutical composition, so as to improve drug safety and reduce related substances , quality controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
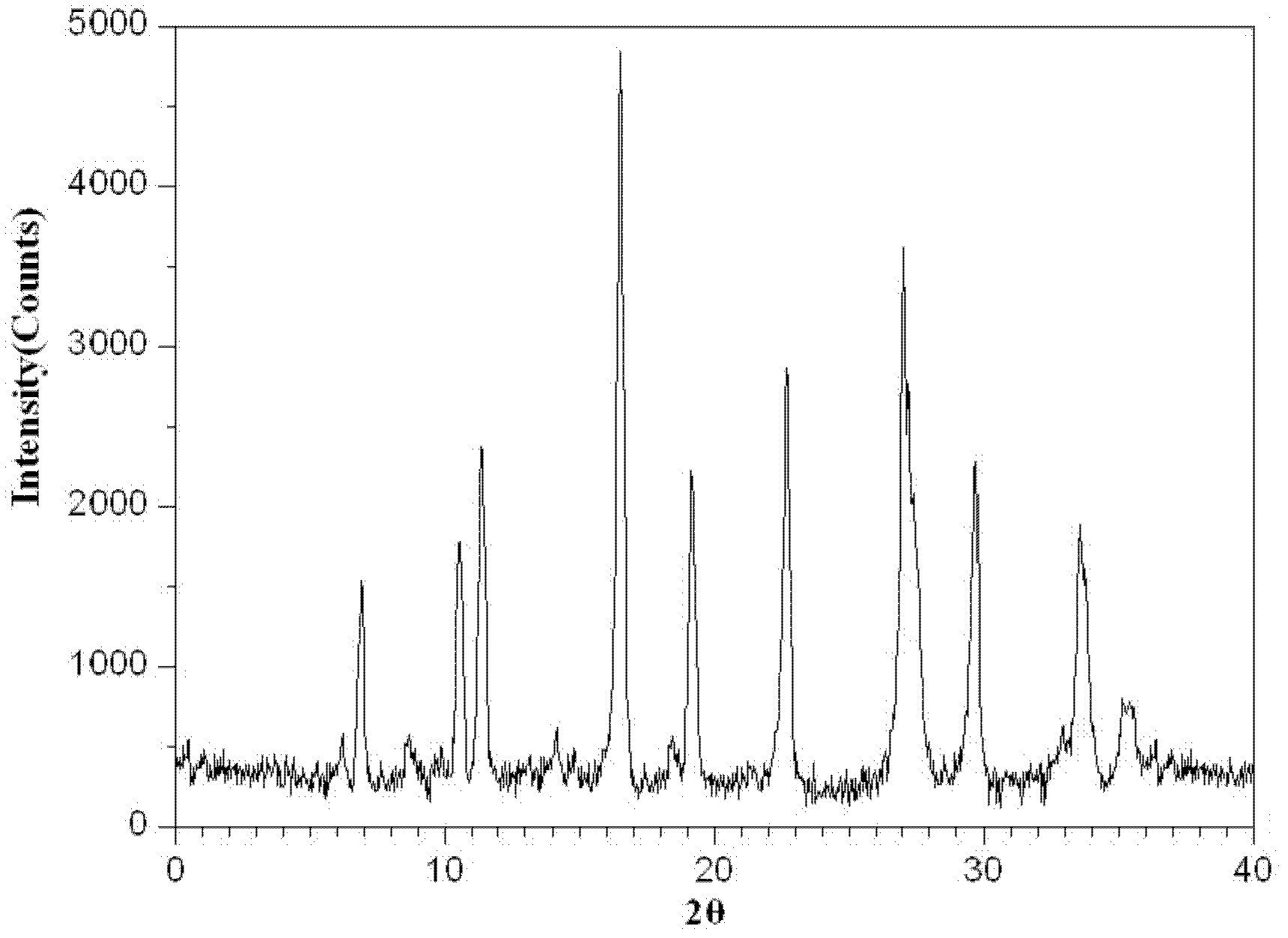
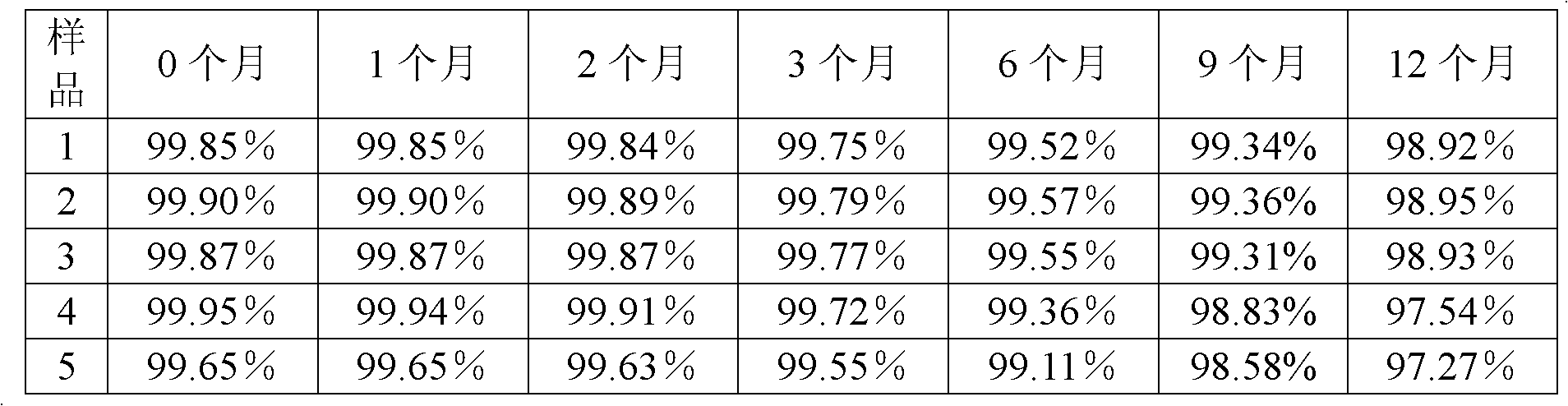
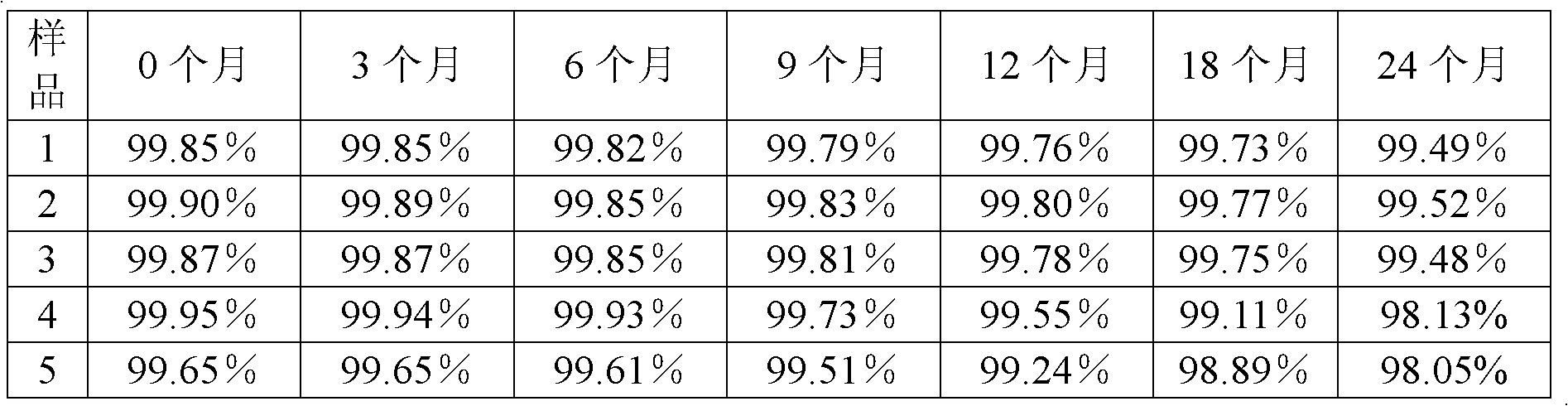
[0040] The preparation method of the tazobactam sodium provided by the present invention is as follows: N, N-dimethylformamide and acetone are formulated into a mixed solvent with a volume ratio of 3:1, 100 g of the tazobactam sodium bulk drug is taken, and N , 850ml of mixed solvent of N-dimethylformamide and acetone, stirred until completely dissolved, and adjusted the pH of the solution to 5.5 with acetic acid. The solution was flowed through a 0.5T DC magnetic field at a speed of 10m / s, and the direction of the magnetic field was perpendicular to the flow direction of the solution. After the solution has undergone a magnetic treatment, add activated carbon to decolorize, filter to obtain a clear solution, add 5100ml cyclohexane to the clear solution, filter to obtain a filter cake, wash the filter cake with acetone, and then dry it under reduced pressure for 4 hours to obtain a white microcrystalline powder. The yield is 90.5%, and the purity is 99.85% by high-performance...
Embodiment 2
[0044] The preparation method of the tazobactam sodium provided by the invention is as follows: N, N-dimethylformamide and acetone are formulated into a mixed solvent with a volume ratio of 1:1, and 100 g of the tazobactam sodium bulk drug is taken, and N , 1050ml of a mixed solvent of N-dimethylformamide and acetone, stirred until completely dissolved, and adjusted the pH of the solution to 5.0 with acetic acid. The solution was flowed through a 0.5T DC magnetic field at a speed of 7m / s, and the direction of the magnetic field was perpendicular to the flow direction of the solution. After the solution is magnetically treated once, add activated carbon for decolorization, filter, and then filter with a 0.22 μm ultrafiltration membrane to obtain a clear solution, add 4200ml cyclohexane to the clear solution, filter to obtain a filter cake, wash the filter cake with acetone, and then Dry under reduced pressure for 2 hours to obtain white microcrystalline powder. The yield was 8...
Embodiment 3
[0048] The preparation method of the tazobactam sodium provided by the present invention is: N,N-dimethylformamide and acetone are formulated into a mixed solvent with a volume ratio of 2:1, take 100g of the tazobactam sodium bulk drug, add N , N-dimethylformamide and acetone mixed solvent 900ml, stirred until completely dissolved, the pH of the solution was adjusted to 5.3 with acetic acid. The solution was flowed through a 0.5 T DC magnetic field at a speed of 15 m / s, and the direction of the magnetic field was perpendicular to the direction of solution flow. After the solution has undergone a magnetic treatment, add activated carbon to decolorize, filter to obtain a clear solution, add 5100ml cyclohexane to the clear solution, filter to obtain a filter cake, wash the filter cake with acetone, and then dry it under reduced pressure for 2 to 4 hours, namely A white microcrystalline powder was obtained. The yield was 89.1%, and the purity was 99.87% by high-performance liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com