Cefoperazone sodium and tazobactam sodium pharmaceutical composition for injection
A technology of tazobactam sodium and cefoperazone sodium, which is applied in the field of medicine, can solve the problems of restricting large-scale production of preparations, poor long-term storage stability, and potential safety hazards in clinical treatment, and achieves good effectiveness and safety, low related substances, and low degradation little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
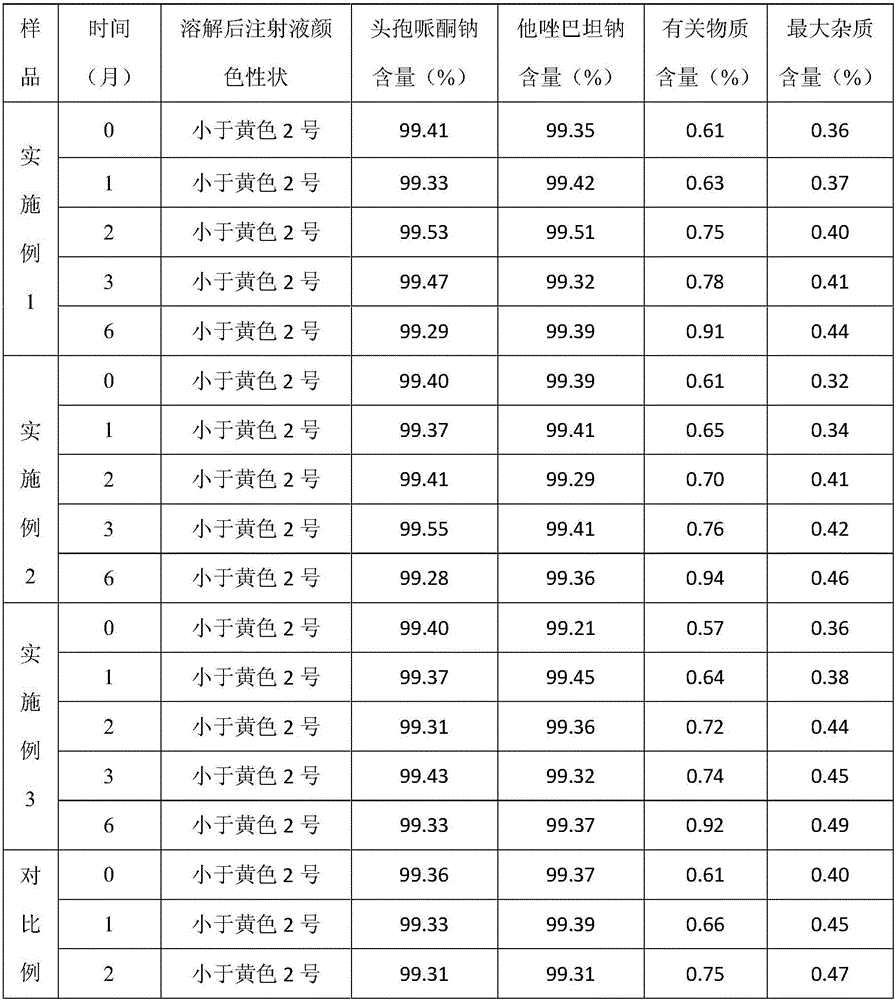
Embodiment 1
[0038] prescription:
[0039] Cefoperazone sodium 120kg (calculated as free cefoperazone)
[0040] Tazobactam sodium 30kg (calculated as free tazobactam)
[0041] Preparation:
[0042] (1) Refining of Tazobactam
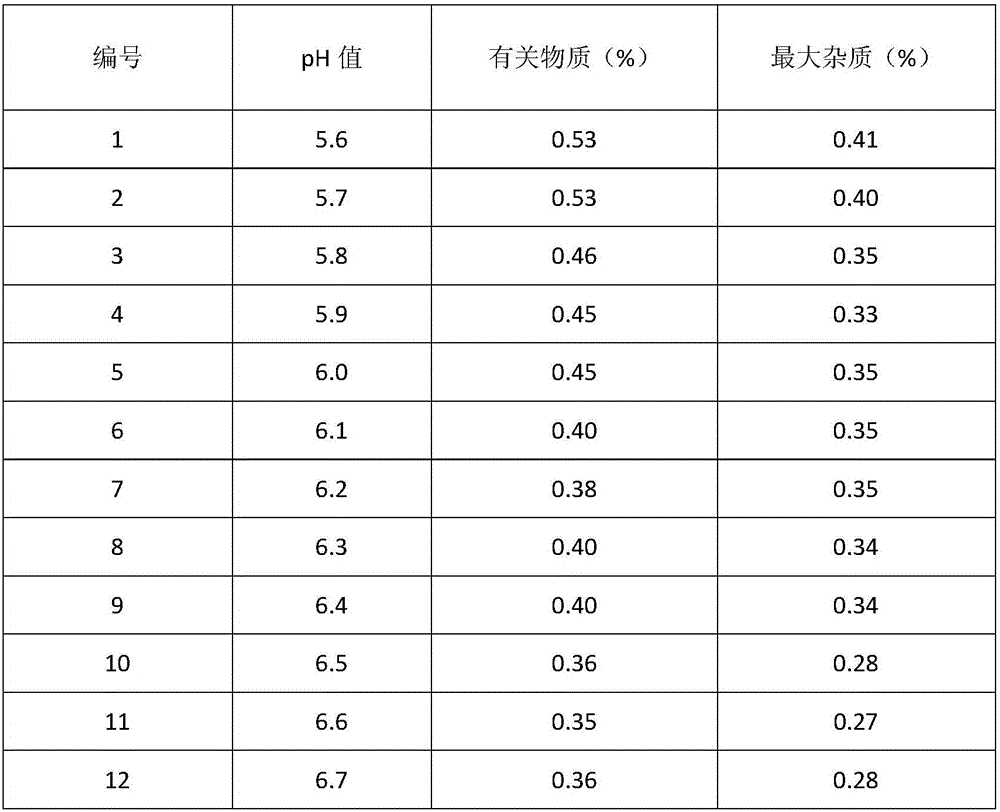
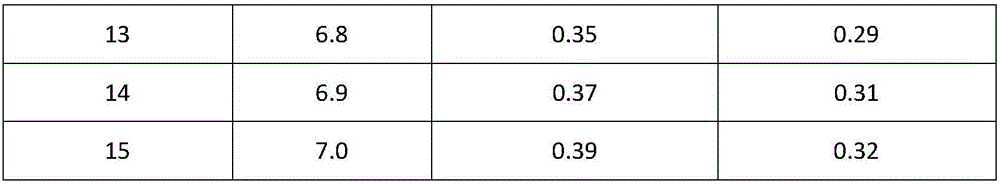
[0043] Add the prescribed amount of tazobactam into the reaction kettle, add water, turn on the stirring and cooling device, and when the temperature reaches 0-5°C, add 8kg of sodium bicarbonate to adjust the pH to 6.2, and add 2Kg of neutral alumina to the reaction solution , stirred for 30 minutes, filtered, and the filtrate was neutralized with concentrated hydrochloric acid at 0-5°C until the pH was 1.5, a large amount of white crystals were precipitated, continued to stir for 2 hours, filtered, washed the filter cake with 0-5°C water for injection, and the filter cake was placed in Vacuum drying at around 45°C yields refined tazobactam.
[0044] (2) Preparation of Tazobactam Sodium
[0045] Add 64Kg of water for injection at 7-10°C to the reaction kettle, f...
Embodiment 2
[0050] prescription:
[0051] Cefoperazone sodium 120kg (calculated as free cefoperazone)
[0052] Tazobactam sodium 30kg (calculated as free tazobactam)
[0053] Preparation:
[0054] (1) Refining of Tazobactam
[0055] Add the prescribed amount of tazobactam into the reaction kettle, add water, turn on the stirring and cooling device, and when the temperature reaches 0-5°C, add 8kg of sodium bicarbonate to adjust the pH to 5.7, and add 2Kg of neutral alumina to the reaction solution , stirred for 30 minutes, filtered, and the filtrate was neutralized with concentrated hydrochloric acid at 0-5°C until the pH was 1.0, a large amount of white crystals were precipitated, continued to stir for 2 hours, filtered, washed the filter cake with 0-5°C water for injection, and the filter cake was placed in Vacuum drying at around 45°C yields refined tazobactam.
[0056] (2) Preparation of Tazobactam Sodium
[0057] Add 64Kg of water for injection at 7-10°C to the reaction kettle, f...
Embodiment 3
[0062] prescription:
[0063] Cefoperazone sodium 120kg (calculated as free cefoperazone)
[0064] Tazobactam sodium 30kg (calculated as free tazobactam)
[0065] Preparation:
[0066] (1) Refining of Tazobactam
[0067] Add the prescribed amount of tazobactam into the reaction kettle, add water, turn on the stirring and cooling device, and when the temperature reaches 0-5°C, add 8kg of sodium bicarbonate to adjust the pH to 6.4, and add 2Kg of neutral alumina to the reaction solution , stirred for 30 minutes, filtered, and the filtrate was neutralized with concentrated hydrochloric acid at 0-5°C until the pH was 2.0, a large amount of white crystals were precipitated, continued to stir for 2 hours, filtered, washed the filter cake with 0-5°C water for injection, and the filter cake was placed in Vacuum drying at around 45°C yields refined tazobactam.
[0068] (2) Preparation of Tazobactam Sodium
[0069] Add 64Kg of water for injection at 7-10°C to the reaction kettle, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com