Medicine composition of piperacillin sodium and tazobactam sodium
A technology of tazobactam sodium and piperacillin sodium is applied in the field of piperacillin sodium tazobactam sodium pharmaceutical composition and compound antibiotics, and can solve the loss of sodium, potassium and calcium ions, increase in procedures, and intravenous infusion Inflammation and other problems, to achieve the effect of high antibacterial activity and high product safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
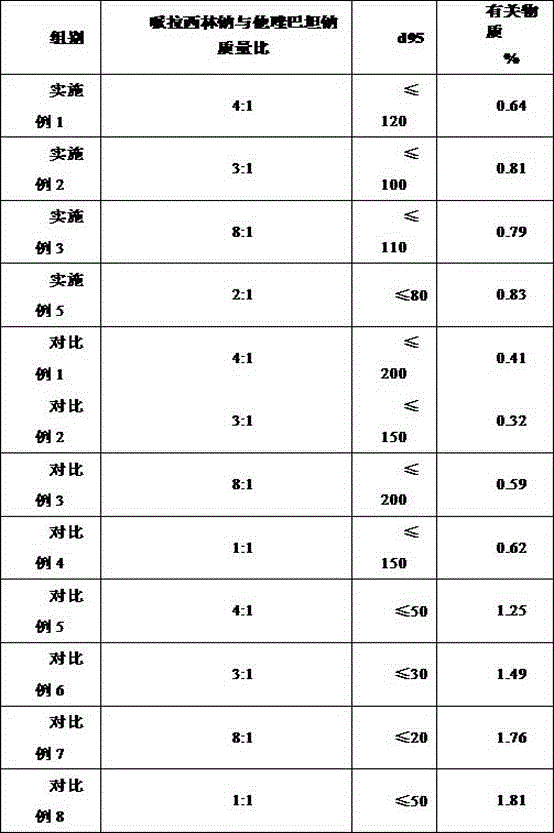
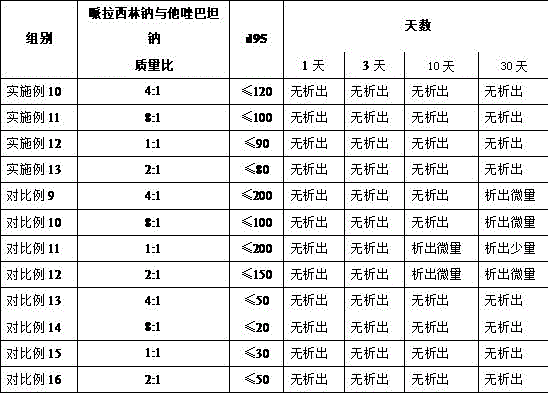
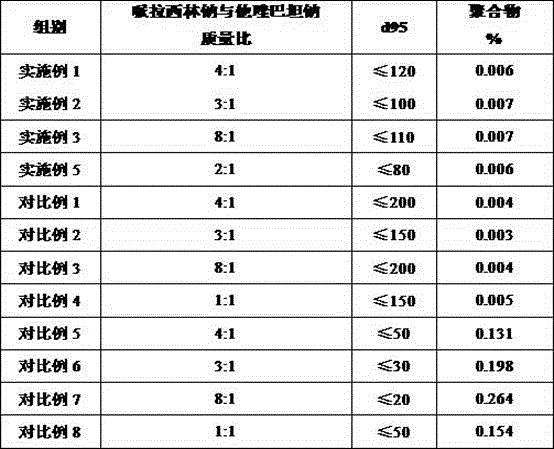
[0019] Weigh 1000.0 g of piperacillin sodium and 250.0 g of tazobactam sodium, and pulverize them in a jet mill until more than 95% of the particles have a particle size of ≤120 mm.
Embodiment 2
[0021] Weigh 1000.0 g of piperacillin sodium and 333.0 g of tazobactam sodium, and pulverize them in a jet mill until more than 95% of the particles have a particle size of ≤100 mm.
Embodiment 3
[0023] Weigh 1000.0 g of piperacillin sodium and 125.0 g of tazobactam sodium, and pulverize them in a jet mill until more than 95% of the particles have a particle size of ≤110 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com