Preparation method of penicillin antibiotic impurity
A penicillin and antibiotic technology, applied in the field of preparation of penicillin antibiotic impurities, can solve problems such as the preparation of penicillin antibiotic impurities that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The invention discloses a method for preparing penicillin-type antibiotic impurities, and those skilled in the art can learn from the contents of this article and appropriately improve the process parameters to realize the method. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0051] All the reagents used in the present invention can be purchased from the market.
Embodiment 1
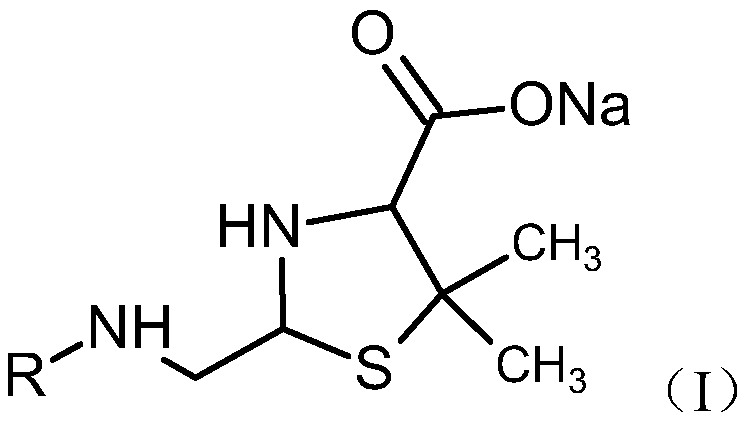
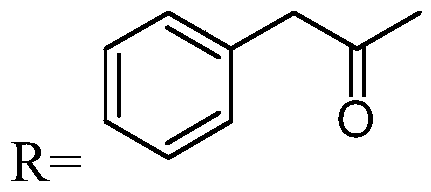
[0053] The preparation method of mezlocillin sodium impurity, this impurity has structure as shown below:
[0054]
[0055] Add 2 g of mezlocillin sodium, 2.5 ml of penicillinase and 25 ml of water to the reaction flask in sequence, and stir at a temperature of 25°C for 30 min. The reaction solution was directly purified by preparative chromatography (eluent: acetonitrile: methanol = 3:1, filler: C18 silica gel) to obtain the target product solution, and then concentrated under reduced pressure to obtain a white solid. Add 10 ml of methanol to the above crude product to dissolve, leave it at room temperature for 2 days, and concentrate under reduced pressure to obtain an off-white powdery solid. HPLC purity: 95.1%. MS [M-H]: 512.1.
[0056] Proton NMR spectrum: 1 H-NMR (500NHz,D 2 O), δ3.803~3.958(m,6H), δ3.378(s,3H), δ5.339-5.347(d,1H), δ7.418~7.502(m,5H), δ4.726-4.735 (d,1H), δ2.055(S,1H), δ3.670(S,1H), δ1.308(S,3H), δ1.393(S,3H).
[0057] Carbon NMR spectrum: 13 C...
Embodiment 2
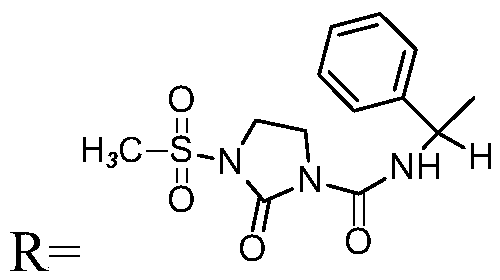
[0059] The preparation method of mezlocillin sodium impurity, this impurity has structure as shown below:
[0060]
[0061] Take 2g of mezlocillin sodium, 2.5ml of cephalosporinase and 25ml of water and add them to the reaction flask in sequence, and stir the reaction at a temperature of 30°C for 60min. The reaction solution was directly purified by preparative chromatography (eluent: acetonitrile: methanol = 3:1, filler: C18 silica gel) to obtain the target product solution, and then concentrated under reduced pressure to obtain a white solid. Add 10 ml of ethanol to the above crude product to dissolve, leave it at room temperature for 2 days, and concentrate under reduced pressure to obtain an off-white powdery solid. HPLC purity: 90.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com