Piperacillin sodium and sulbactam sodium drug composite microsphere injection
A technology of piperacillin sodium sulbactam sodium and cillin sodium sulbactam sodium, which is applied in the field of medicine and can solve the problems of large particle distribution range, large burst release, and poor drug stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
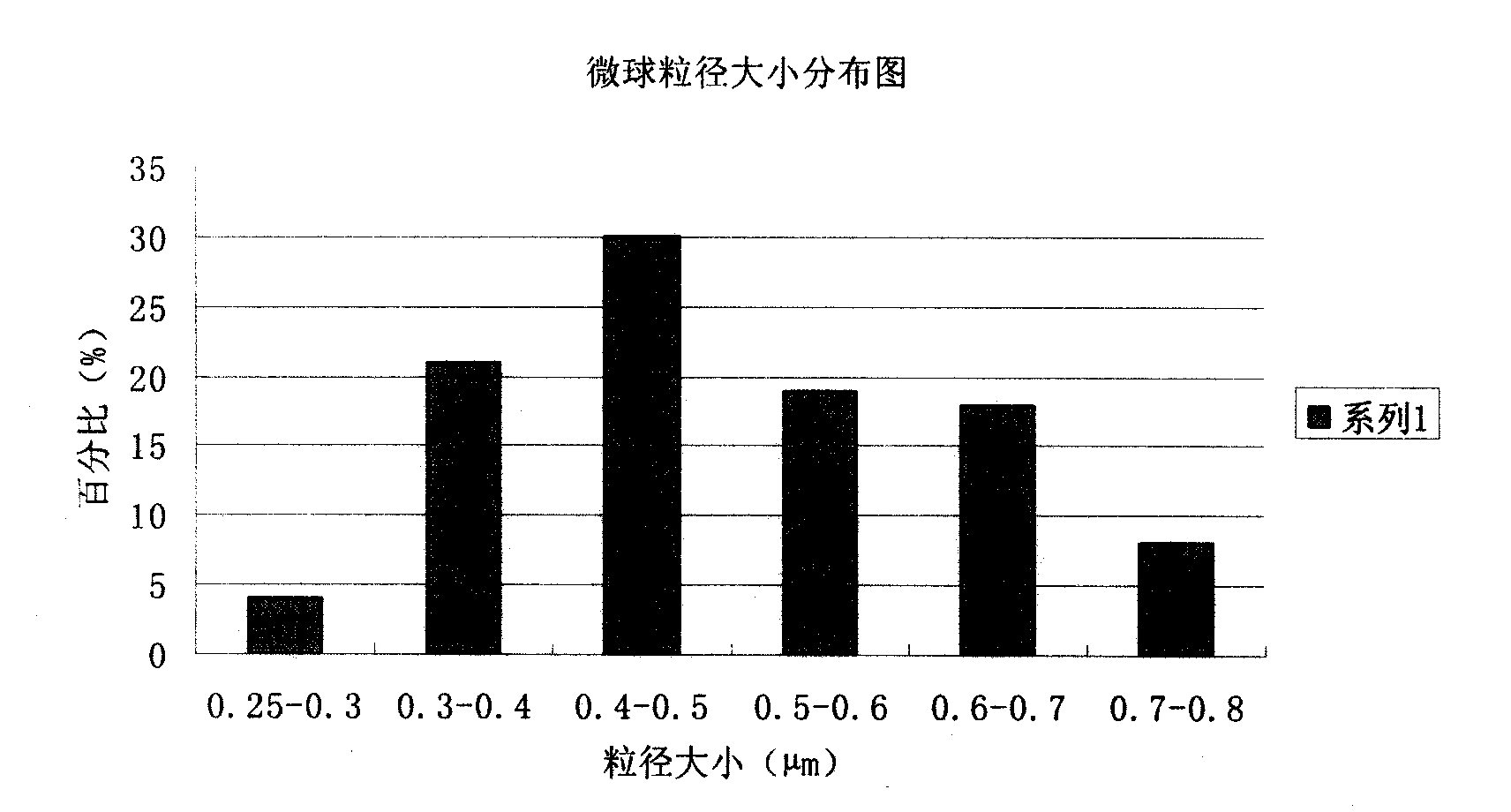
[0063] The preparation of embodiment 1 piperacillin sodium sulbactam sodium pharmaceutical composition microsphere injection
[0064] Prescription: (100 bottles)
[0065] Piperacillin Sodium 40g
[0067] Albumin 60g
[0068] Poloxamer 188 30g
[0069] PEG600 50g
[0070] Mannitol 20g
[0071] making process:
[0072] (1) 40g piperacillin sodium, 10g sulbactam sodium, 50g PEG600, 20g mannitol, 30g poloxamer 188 and 60g albumin were dissolved in 1000ml water for injection, filtered to obtain a clear solution;
[0073] (2) Pour the above solution into the spray dryer, adjust the spray conditions: the inlet temperature is 70°C, the outlet temperature is about 35°C, the nozzle size is 0.5mm, the spray flow rate is 6ml / min, the compressed air velocity is 10L / min, spray dry , to obtain white microspheres with uniform shape;
[0074] (3) The obtained microspheres were finally heated and solidified at a temperature of 100° C. for 8 hours, and distr...
Embodiment 2
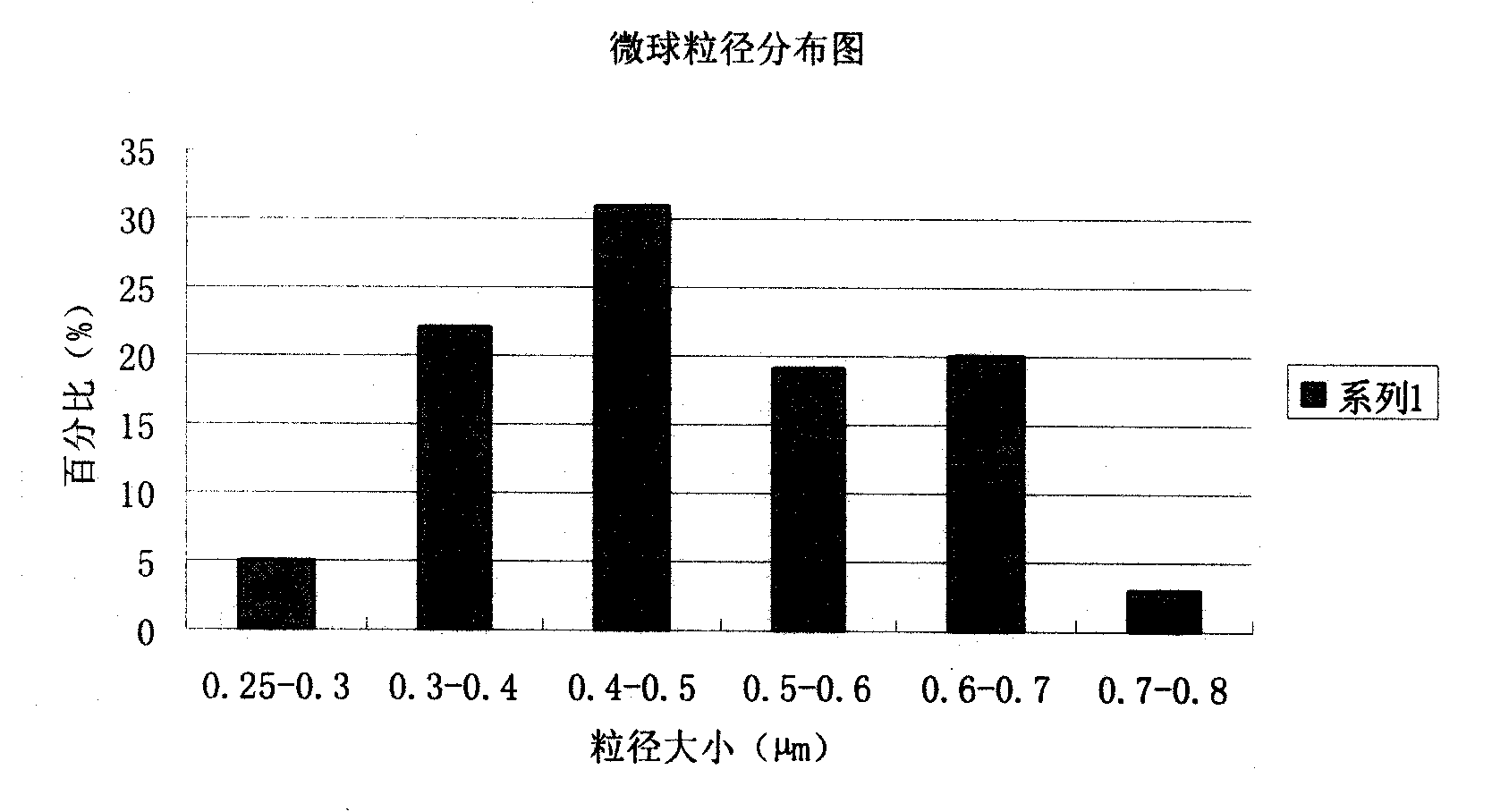
[0077] The preparation of embodiment 2 piperacillin sodium sulbactam sodium pharmaceutical composition microsphere injection
[0078] Prescription: (100 bottles)
[0079] Piperacillin Sodium 20g
[0080] Sulbactam Sodium 10g
[0081] Albumin 80g
[0082] Poloxamer 188 40g
[0083] PEG600 60g
[0084] Mannitol 40g
[0085] making process:
[0086] (1) Dissolve 20g of piperacillin sodium, 10g of sulbactam sodium, 60g of PEG600, 40g of mannitol, 80g of albumin and 40g of poloxamer 188 in 2000ml of water for injection, and filter to obtain a clear solution;
[0087](2) Pour the above solution into a spray dryer and adjust the spray conditions: the inlet temperature is 80°C, the outlet temperature is about 40°C, the size of the nozzle is 1mm, the spray flow rate is 15ml / min, the compressed air velocity is 20L / min, spray dry, Obtain white microspheres with uniform shape;
[0088] (3) The obtained microspheres were finally heated and solidified at a temperature of 80° C. for ...
Embodiment 3
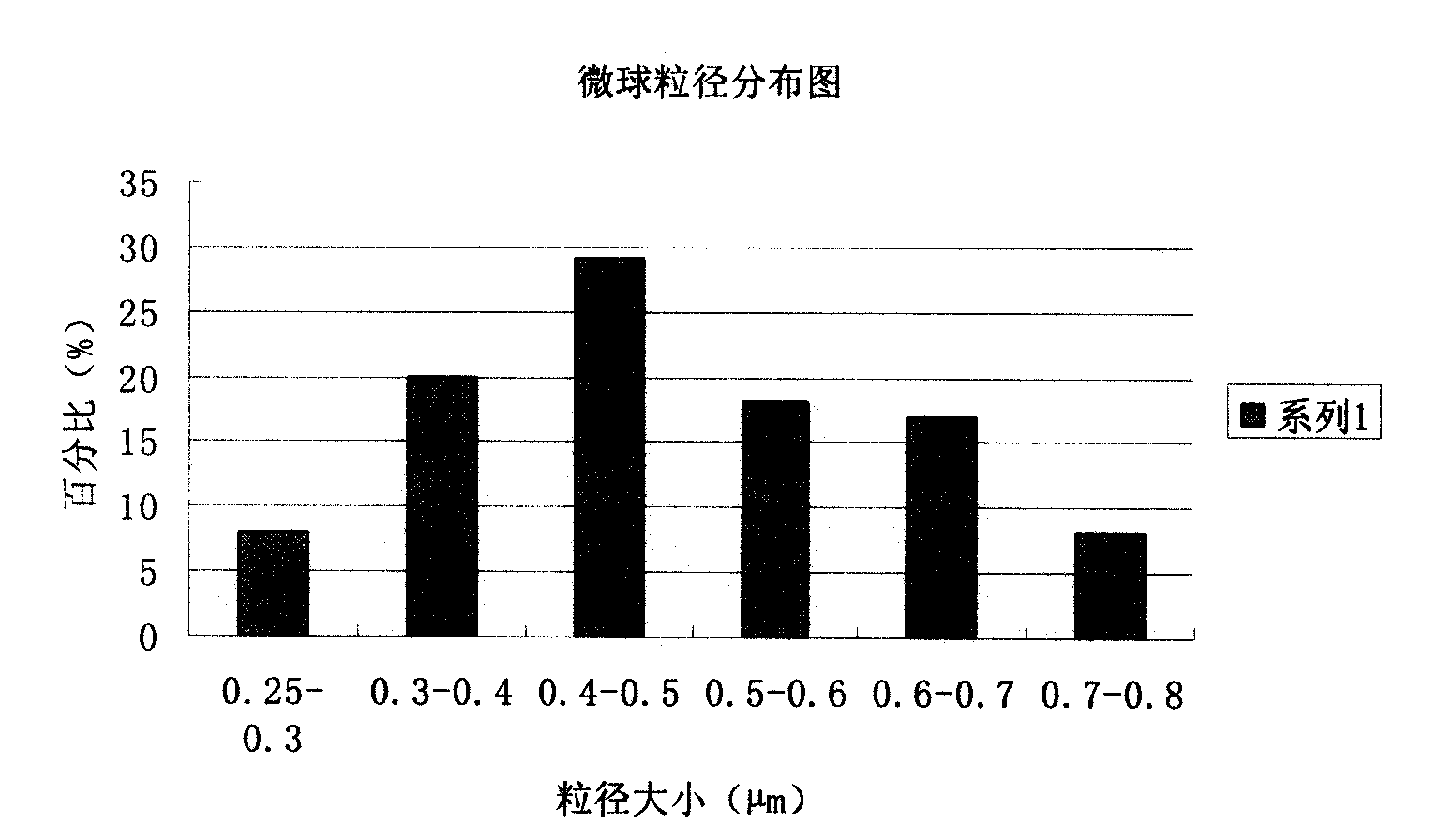
[0091] The preparation of embodiment 3 piperacillin sodium sulbactam sodium pharmaceutical composition microspheres
[0092] Prescription: (100 bottles)
[0093] Piperacillin Sodium 40g
[0094] Sulbactam Sodium 10g
[0095] Albumin 100g
[0096] Poloxamer 188 50g
[0097] PEG60070g
[0098] Mannitol 50g
[0099] making process:
[0100] (1) Dissolve 40g of piperacillin sodium, 10g of sulbactam sodium, 70g of PEG600, 50g of mannitol, 100g of albumin and 50g of poloxamer 188 in 2000ml of water for injection, and filter to obtain a clear solution;
[0101] (2) Pour the above solution into the spray dryer, adjust the spray conditions: the inlet temperature is 70°C, the outlet temperature is about 45°C, the nozzle size is 0.8mm, the spray flow rate is 12ml / min, the compressed air velocity is 15L / min, spray dry , to obtain white microspheres with uniform shape;
[0102] (3) The obtained microspheres were finally heated and solidified at a temperature of 120° C. for 4 hours,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com