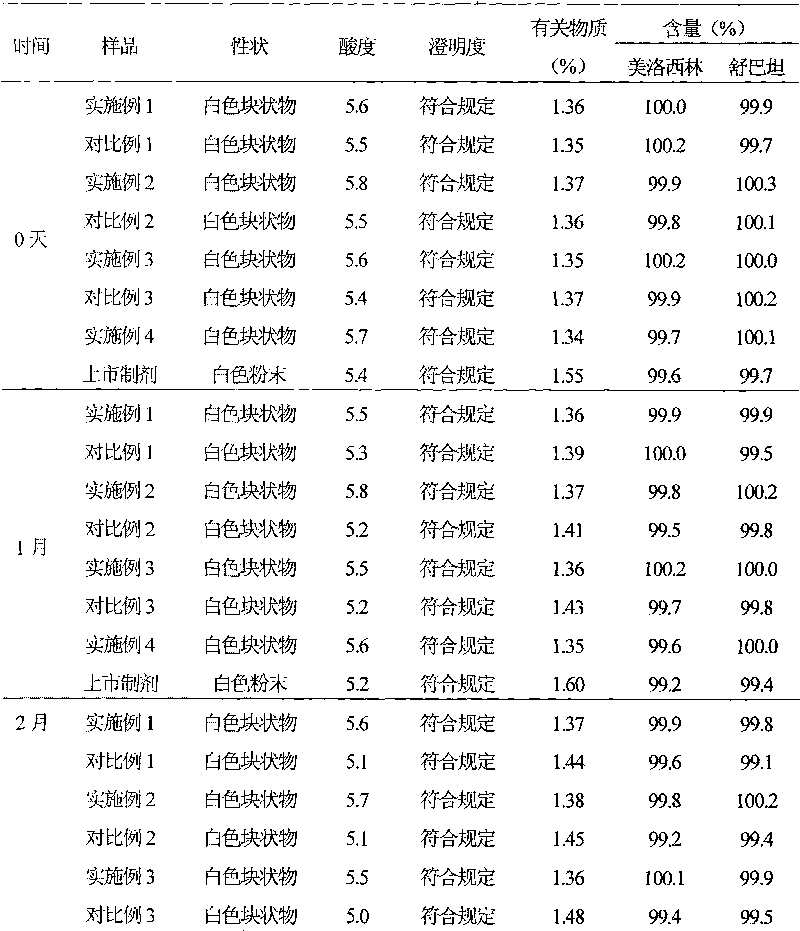
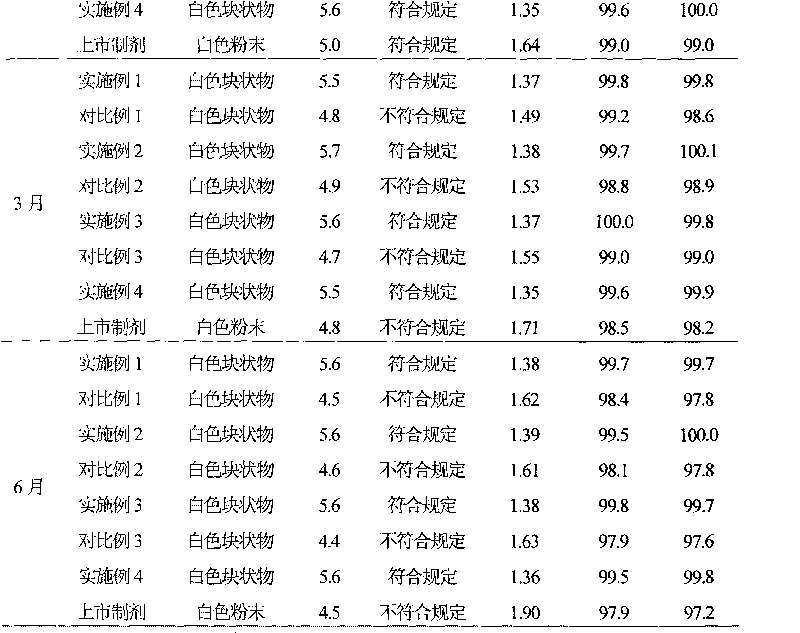
Medicinal-composition suspension powder injection with mezlocillin sodium and sulbactam sodium, and novel application thereof
The technology of mezlocillin sodium and sulbactam sodium and mezlocillin sodium is applied in a new application field in the preparation of a drug for preventing postoperative infection after appendicitis, which can solve the problems of poor stability of mezlocillin sodium and sulbactam sodium, and improve the The effect of drug therapeutic index, improved stability and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of Mezlocillin Sodium Sulbactam Sodium Suspension Powder Injection
[0050] Prescription (100 bottles):
[0051] Mezlocillin Sodium 300g
[0053] Tween 80 375g
[0054] Cholesterol 150g
[0055] Sodium deoxycholate 75g
[0056] Mannitol 225g
[0057] Lactose 75g
[0058] Preparation Process
[0059] (1) Add 375g of Tween 80, 150g of cholesterol and 75g of sodium deoxycholate into 5500ml of water for injection, then add 300g of mezlocillin sodium and 75g of sulbactam sodium and mix evenly, heat and stir in a water bath at 80°C until molten;
[0060] (2) Keeping the above liquid at 70-90°C for 10 minutes with a tissue masher to shear and stir at a speed of 10,000 r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 5 times to obtain an emulsion;
[0061] (3) Add 225g of mannitol and 75g of lactose to the emulsion, dissolve, filter, subpackage, and freez...
Embodiment 2
[0074] Example 2 Preparation of Mezlocillin Sodium Sulbactam Sodium Suspension Powder Injection
[0075] Prescription (100 bottles):
[0076] Mezlocillin Sodium 200g
[0077] Sulbactam Sodium 50g
[0078] Tween 80 750g
[0079] Cholesterol 400g
[0080] Sodium deoxycholate 500g
[0081] Glucose 500g
[0082] Trehalose 500g
[0083] Preparation Process
[0084] (1) Add 750g of Tween 80, 400g of cholesterol and 500g of sodium deoxycholate into 9000ml of water for injection, then add 200g of mezlocillin sodium and 50g of sulbactam sodium and mix evenly, heat and stir in a water bath at 70°C until molten;
[0085] (2) Heat the above liquid at 70-90°C and use a tissue masher to shear and stir for 20 minutes at a speed of 15000r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 4 times to obtain an emulsion;
[0086] (3) Add 500 g of glucose and 500 g of trehalose to the emulsion, dissolve, filter, subpackage, an...
Embodiment 3
[0097] Example 3 Preparation of Mezlocillin Sodium Sulbactam Sodium Suspension Powder Injection
[0098] Prescription (100 bottles):
[0099] Mezlocillin Sodium 100g
[0100] Sulbactam Sodium 25g
[0101] Tween 80 250g
[0102] Cholesterol 125g
[0103] Sodium deoxycholate 150g
[0104] Mannitol 214.3g
[0105] Glycine 85.7g
[0106] Preparation Process
[0107] (1) Add 250g of Tween 80, 125g of cholesterol and 150g of sodium deoxycholate into 4000ml of water for injection, then add 100g of mezlocillin sodium and 25g of sulbactam sodium and mix evenly, heat and stir in a water bath at 90°C until molten;
[0108] (2) The above liquid was kept warm at 70-90°C and was sheared and stirred for 20 minutes with a tissue masher at a speed of 13000r / min to obtain a primary emulsion, which was then circulated and emulsified by a high-pressure homogenizer for 5 times to obtain an emulsion;
[0109] (3) Add 214.3 g of mannitol and 85.7 g of glycine to the emulsion, dissolve, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com