Preparation of cefoperazone and sulbactam sodium mixed powder
A technology of cefoperazone sodium and sulbactam sodium, applied in the field of medicine, can solve the problems of the density of cefoperazone sodium and sulbactam sodium, the influence of powder fluidity and the uniformity of mixed powder, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0008] A preparation method of cefoperazone sodium / sulbactam sodium mixed powder is characterized in that cefoperazone acid, sulbactam acid and salt-forming agent are reacted in water to form a salt and then dissolved in water, and a uniform product is obtained by a freeze-drying process, Specific steps are as follows:
[0009] (1) Control the temperature at 0-10°C, make a 20-40%wt aqueous solution with a weight ratio of cefoperazone acid to sulbactam acid (according to pure calculation) of 0.9-2.1:1, and adjust the pH of the feed solution with a salt-forming agent To 6.0-6.5, filter with a 0.22μm filter membrane into a freeze-drying box;
[0010] (2) Cool down to -40~-50°C or lower within 2-3 hours, and keep it for 2-3 hours;
[0011] (3) Turn on the vacuum and reach the pressure of 0.10mbar~0.30mbar for vacuum drying, raise the set temperature to 5~15°C and keep it for at least 2 hours, then raise the set temperature to 30~40°C and keep it for at least 10 hours Guarantee t...
Embodiment 1
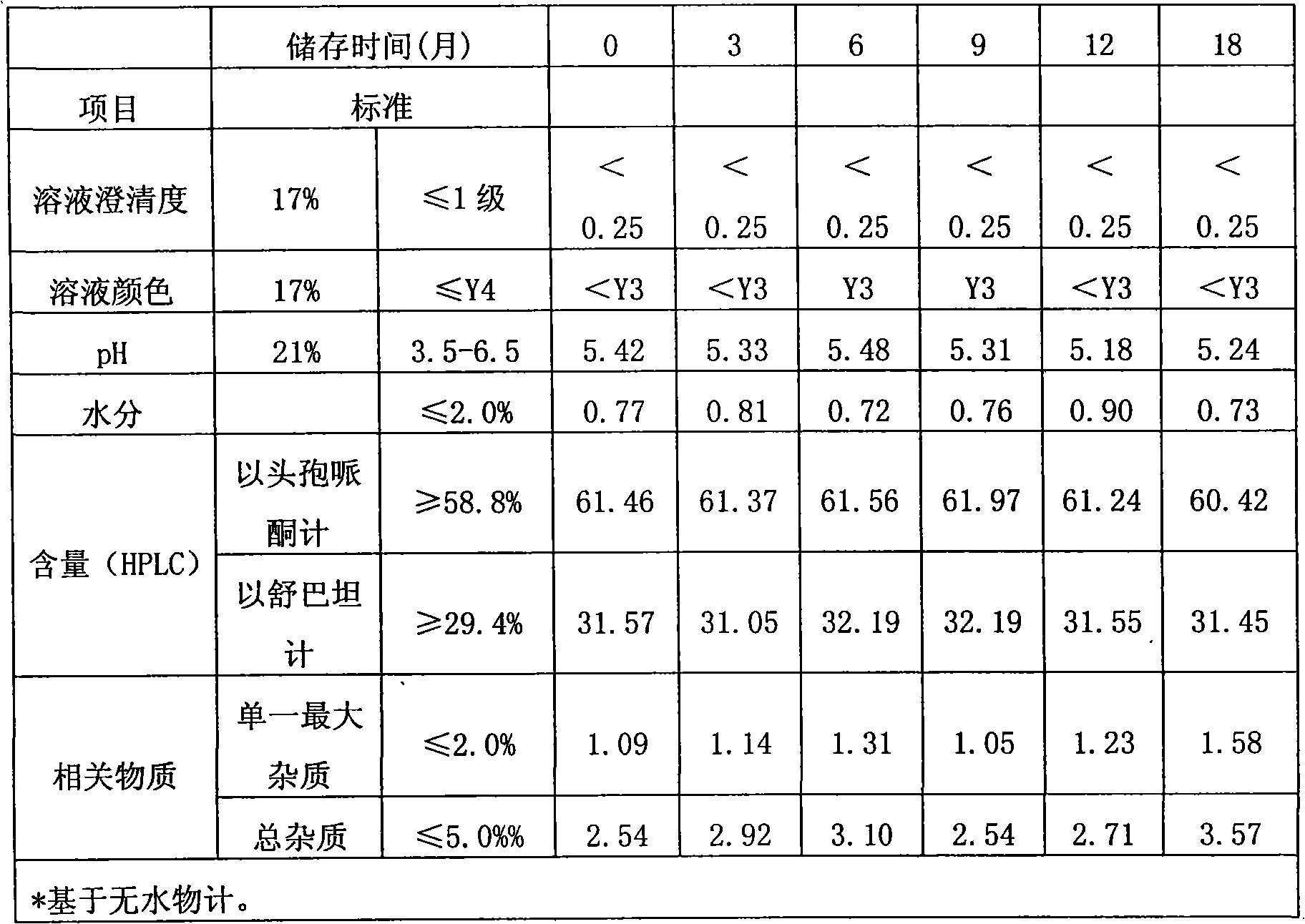
[0026] Control the temperature at 0-5°C, add 74g of cefoperazone acid and 35g of sulbactam acid to 200ml of water for injection, add 21.94g of sodium bicarbonate to adjust the pH of the feed solution to 6.37, and filter it through a 0.22μm filter membrane into a freeze-drying box. Cool down to -45°C in 2-3 hours and keep for 2 hours. Turn on the vacuum and reach 0.10-0.30mbar for vacuum drying, raise the set temperature to 10°C and keep it for 3 hours, then raise the set temperature to 40°C and keep it for 12 hours to completely sublimate the water. The temperature was raised to 55°C and dried for 10 hours, and the water content was detected to be 0.76%, and the temperature was lowered to 30°C to obtain 108.5 g of the product.
Embodiment 2
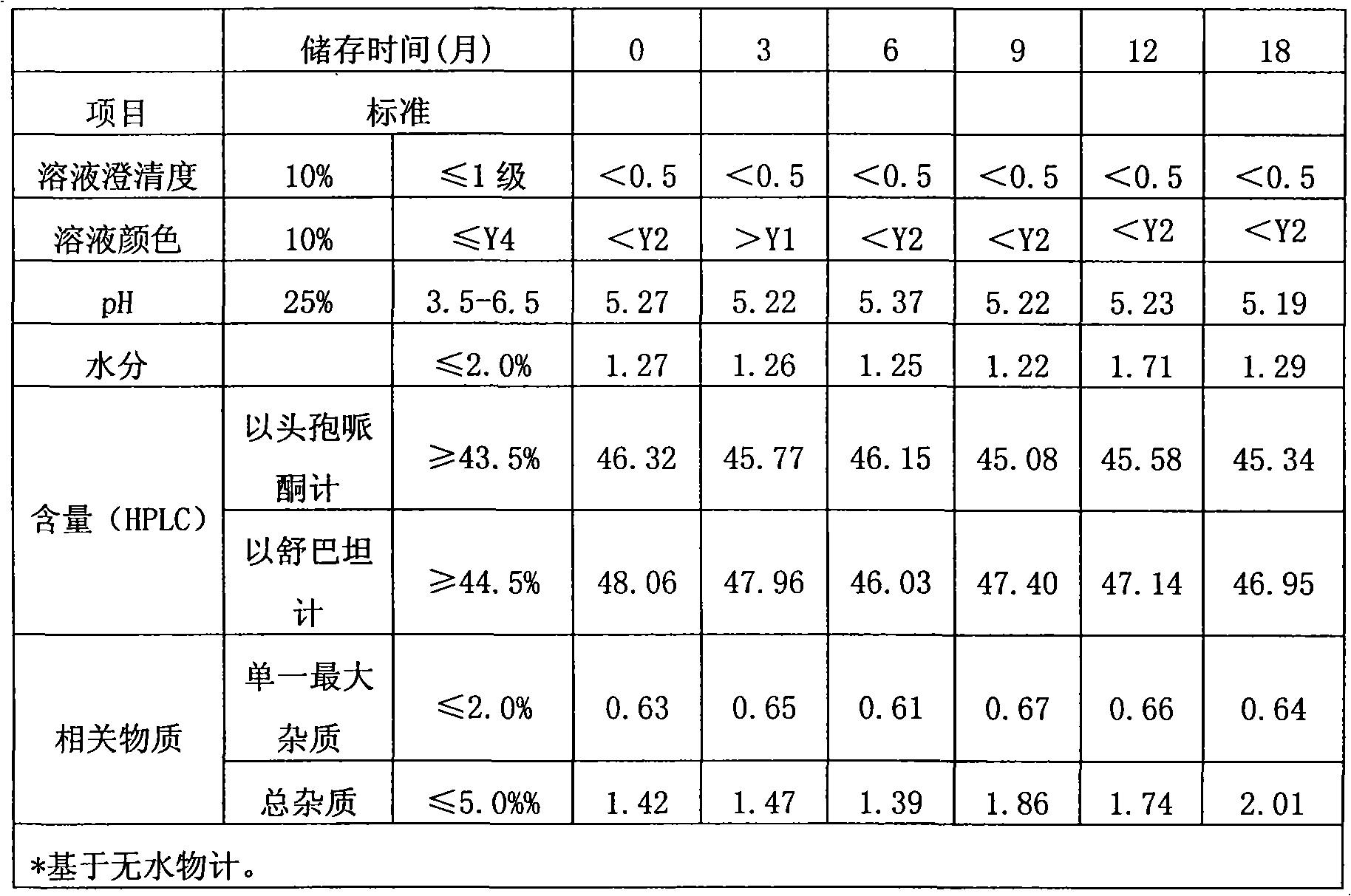
[0028] Control the temperature at 5-10°C, add 42g of cefoperazone acid and 20g of sulbactam acid to 130ml of water for injection, add 8.07g of sodium carbonate to adjust the pH of the feed solution to 6.13, and filter it through a 0.22μm filter membrane into a freeze-drying box. Cool down to -42°C in 2-3 hours and keep for 3 hours. Turn on the vacuum and reach 0.10-0.30mbar for vacuum drying, raise the set temperature to 15°C and keep it for 2 hours, then raise the set temperature to 32°C and keep it for 14 hours to ensure the water sublimation is complete. The temperature was raised to 50°C and dried for 12 hours, and the moisture was detected to be 1.06%, and the temperature was lowered to 30°C to obtain 60.83 g of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com