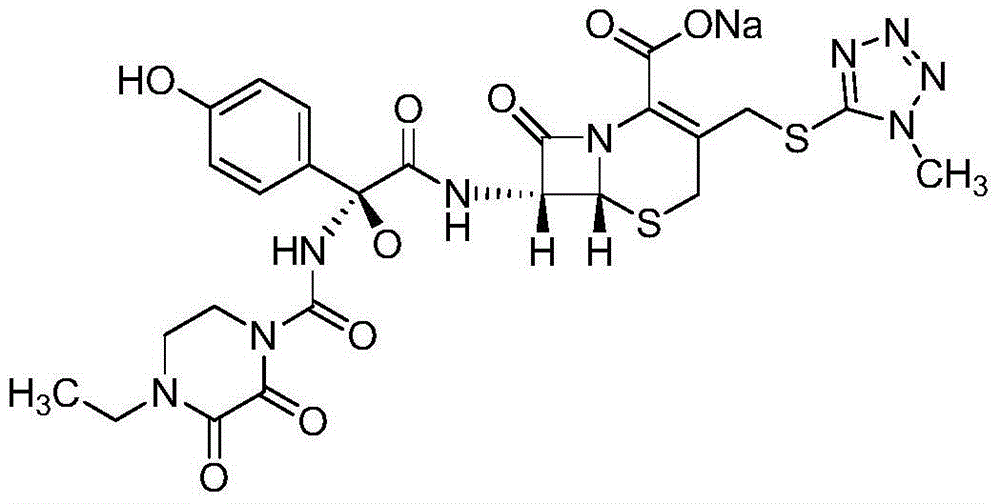
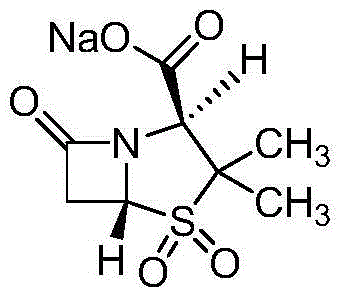
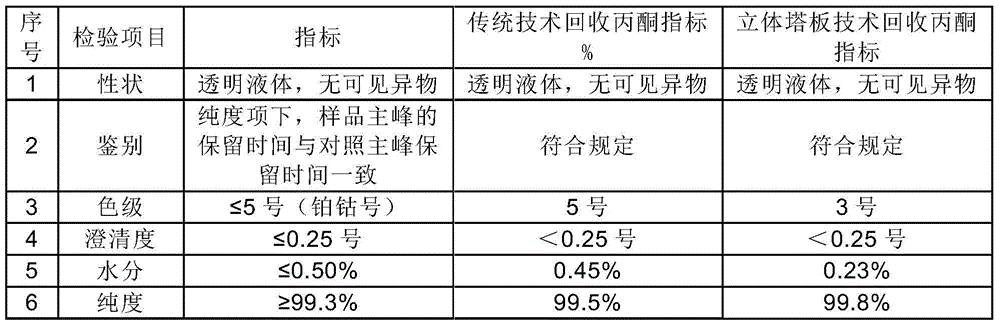
Preparation method of cefoperazone sodium and sulbactam sodium powder injection for injection
A technology of cefoperazone sodium sulbactam sodium powder and cefoperazone sodium, which is applied in the field of medicine, can solve the problems of low solvent content, increased process difficulty, low mass transfer efficiency, etc., and achieve the effect of reduced impurity content and good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 (adopting traditional technology to reclaim solvent)
[0030] (1) Preparation of cefoperazone sodium: add 200kg cefoperazone acid, 200L purified water, 400L of traditional technology to reclaim acetone in the dissolving tank, add 30kg of sodium bicarbonate, control the temperature at 15°C, stir and dissolve, then filter into the crystallization tank , control the temperature at 18°C, continue to add 3600L of acetone recovered by traditional technology for crystallization, grow crystals at 0-5°C, filter, and vacuum-dry at 45°C to obtain the finished product of cefoperazone sodium.
[0031] (2) Preparation of sulbactam sodium: add 800L of ethanol reclaimed by traditional technology, 200kg of sulbactam acid, 200kg of sodium acetate, 500L of ethanol reclaimed by traditional technology in a dissolving tank, stir at 40°C for temperature control, dissolve and filter, then temperature control for 40°C Under the condition of 1500°C, add 1500L of ethanol recovered by...
Embodiment 2
[0035] Embodiment 2 (adopt traditional technology to reclaim solvent)
[0036] (1) The preparation of cefoperazone sodium: add 400kg cefoperazone acid in dissolving tank, 400L purified water, the traditional technology of 600L reclaims acetone, adds sodium bicarbonate 70kg, after stirring and dissolving, filter in the crystallization tank, continue to add traditional technology Recover 5000L of acetone for crystallization, grow the crystal, filter, and dry at 40-50°C to obtain the finished product of cefoperazone sodium.
[0037] (2) Preparation of sulbactam sodium: add 800L of ethanol recovered by traditional technology, 200kg of sulbactam acid, 200kg of sodium acetate, 500L of ethanol recovered by traditional technology in a dissolving tank, stir, dissolve and filter, then add 1500L of ethanol recovered by traditional technology to carry out Crystallize, filter and wash, and dry at 40-50°C to obtain sulbactam sodium.
[0038] (3) Preparation of mixed powder of cefoperazone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com