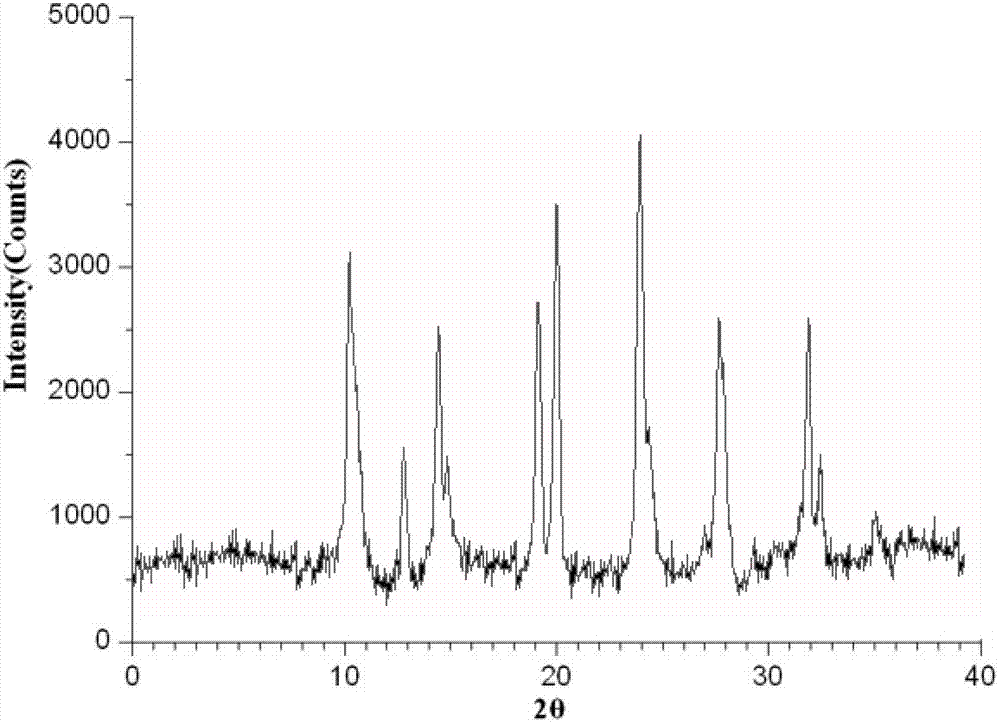
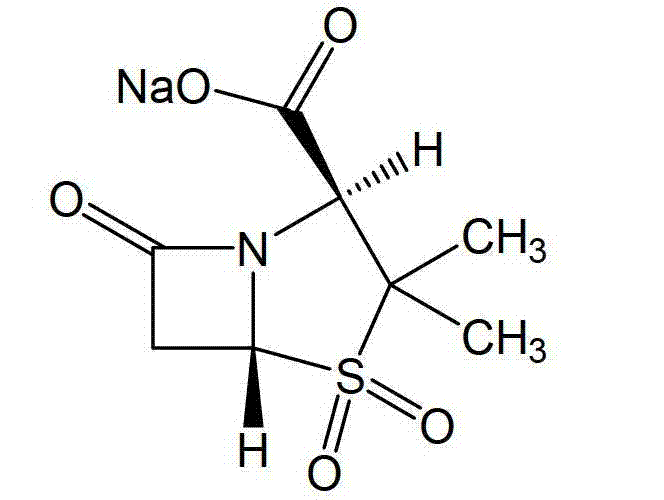
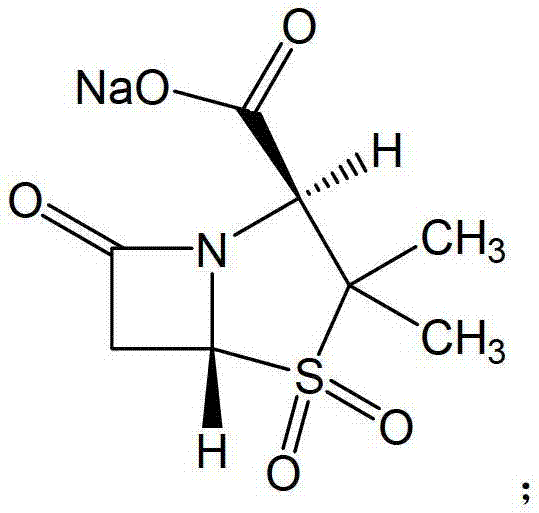
Sulbactam sodium compound and medical composition of sulbactam sodium compound and mezlocillin sodium
A technology of mezlocillin sodium and sulbactam sodium, which is applied in the field of medicine, can solve the problems of high impurity content, poor storage stability, and poor use safety, and achieve high safety performance, low impurity content, and good storage stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of sulbactam sodium compound
[0037] N,N-dimethylformamide and water are formulated into a mixed solvent at a volume ratio of 3:1, and the raw material drug of sulbactam sodium is taken, and the mixed solvent of N,N-dimethylformamide and water is added, and the The ratio of the volume of the mixed solvent to the mass of sulbactam sodium is 5ml:1g, heat up to 70°C, stir until completely dissolved, keep warm, adjust the pH of the solution to 5.5, and flow the solution through 0.5T at a speed of 5m / s DC magnetic field, the direction of the magnetic field is perpendicular to the flow direction of the solution, add activated carbon to the solution after magnetic treatment for decolorization, the amount of activated carbon is 0.3% (g / ml) of the volume of the mixed solvent, stir for 30min, filter to obtain a clear solution, and pour it into the clear solution Add ethanol to the solution, the volume ratio of the ethanol to the mixed solvent is 5:1, filter to obtain...
Embodiment 2
[0040] Preparation of sulbactam sodium compound
[0041]N,N-dimethylformamide and water are prepared into a mixed solvent at a volume ratio of 1:1, and the raw material drug of sulbactam sodium is taken, and the mixed solvent of N,N-dimethylformamide and water is added, and the The ratio of the volume of the mixed solvent to the mass of sulbactam sodium is 3ml:1g, heat up to 60°C, stir until completely dissolved, keep warm, adjust the pH of the solution to 5.0, and flow the solution through 0.5T at a speed of 10m / s DC magnetic field, the direction of the magnetic field is perpendicular to the flow direction of the solution, add activated carbon to the solution after magnetic treatment for decolorization, the amount of activated carbon is 0.3% (g / ml) of the volume of the mixed solvent, stir for 30min, filter to obtain a clear solution, and pour it into the clear solution Add ethanol to the solution, the volume ratio of the ethanol to the mixed solvent is 10:1, filter to obtain ...
Embodiment 3
[0044] Preparation of Mezlocillin Sodium and Sulbactam Sodium Powder Injection
[0045] Take by weighing mezlocillin sodium and the sulbactam sodium that embodiment 1 prepares under aseptic condition, wherein the mass ratio of mezlocillin sodium and sulbactam sodium is 4:1, is placed in the solid powder mixer and evenly mixed, The raw materials obtained are transferred to the aseptic preparation workshop, where they are precisely measured and packaged. Each bottle contains 0.5 g of sulbactam sodium, which is stoppered and capped. The finished product is packaged and put into storage for inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com