Vinpocetine compound and drug composition thereof
A technology of vinpocetine and its composition, which is applied in the field of vinpocetine compounds, can solve the problems of poor storage stability of vinpocetine, achieve the effects of improving storage stability, less impurity content, and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
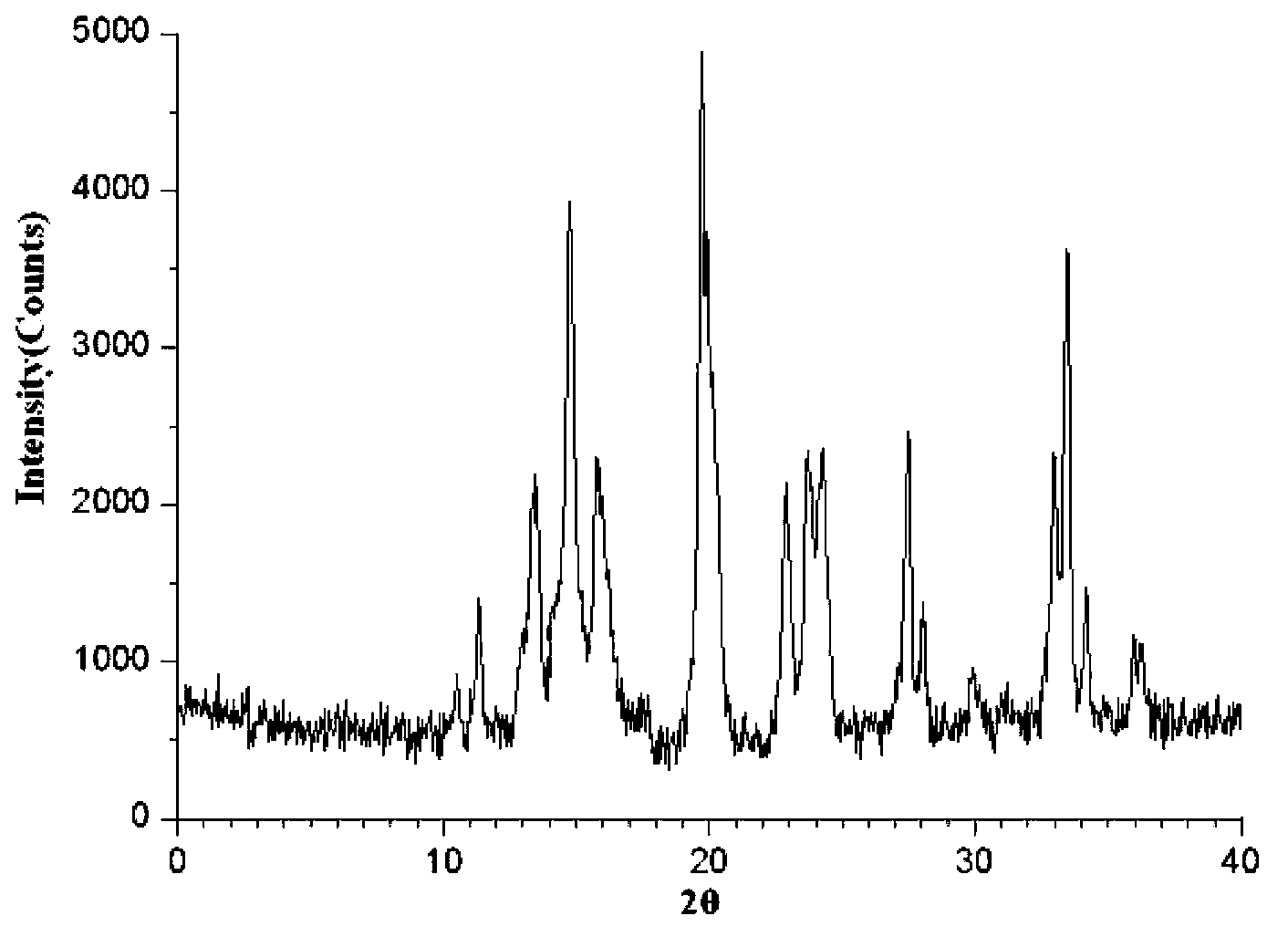
Image
Examples
Embodiment 1
[0039] Preparation of Vinpocetine Compounds
[0040] N,N-dimethylformamide and ethanol are formulated into a mixed solvent at a volume ratio of 2:1, and the raw material drug of vinpocetine is added to a mixed solvent of N,N-methylformamide and ethanol, and the mixed solvent The ratio of the volume of vinpocetine to the mass of vinpocetine is 4ml: 1g, heat up to 60°C, stir until completely dissolved, keep warm, adjust the pH of the solution to 5.5, add activated carbon for decolorization, the amount of activated carbon is 0.3% of the volume of the mixed solvent (g / ml), stirred for 30 minutes, filtered to obtain a clear solution, moved the clear solution into a reaction kettle, placed it in an oven at 100°C for 48 hours, and let it stand to cool down naturally. When the temperature dropped below 70°C, open the kettle and pour into the Add ice water to the solution, the volume ratio of the ice water to the mixed solvent is 15:1, filter to obtain a filter cake, wash the filter ca...
Embodiment 2
[0043] Preparation of Vinpocetine Compounds
[0044]N,N-dimethylformamide and ethanol are formulated into a mixed solvent at a volume ratio of 4:1, and the raw material drug of vinpocetine is added to a mixed solvent of N,N-methylformamide and ethanol, and the mixed solvent The ratio of the volume of Vinpocetine to the mass of Vinpocetine is 6ml: 1g, heat up to 70°C, stir until completely dissolved, keep warm, adjust the pH of the solution to 6.0, add activated carbon to decolorize, the amount of activated carbon is 0.3% of the volume of the mixed solvent (g / ml), stirred for 30 minutes, and filtered to obtain a clear solution. Move the clear solution into a reaction kettle, place it in an oven at 120°C for 12 hours, and let it cool down naturally. Add ice water to the solution, the volume ratio of the ice water to the mixed solvent is 5:1, filter to obtain a filter cake, wash the filter cake with ethanol at 5°C for 3 times, and then dry under reduced pressure for 2 hours to ob...
Embodiment 3
[0047] Preparation of Vinpocetine Sterile Powder Injection
[0048] Weigh 10 parts of vinpocetine and 5 parts of ascorbic acid prepared in Example 1 under sterile conditions, place them in a solid powder mixer and mix them evenly, and transfer the obtained raw materials to a sterile preparation workshop for precise metering and packaging. Each bottle contains Changchun Xitine 0.5g, stoppered and capped, the finished product is packaged for storage and sent for inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com