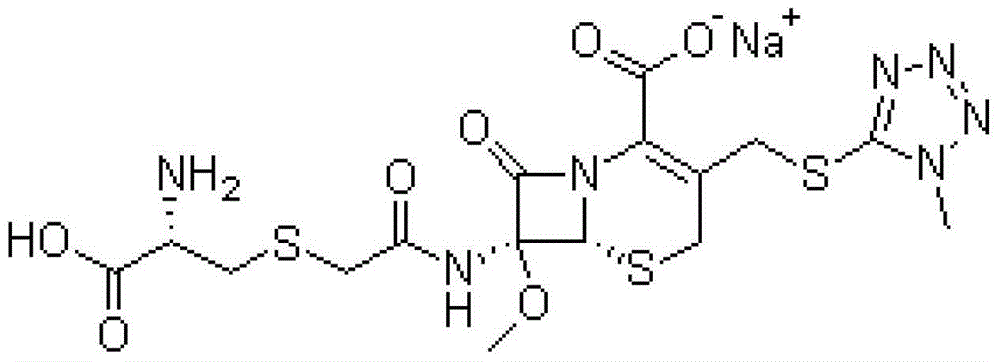
Cefminox sodium compound as well as preparation method and pharmaceutical composition of cefminox sodium compound
A technology of cefminox sodium and compound, applied in the field of medicine, can solve the problems of slow dissolution rate of cefminox sodium crystal, high bioavailability, good solubility, etc., and achieves improved storage stability, good storage stability and safety high performance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of cefminox sodium compound:
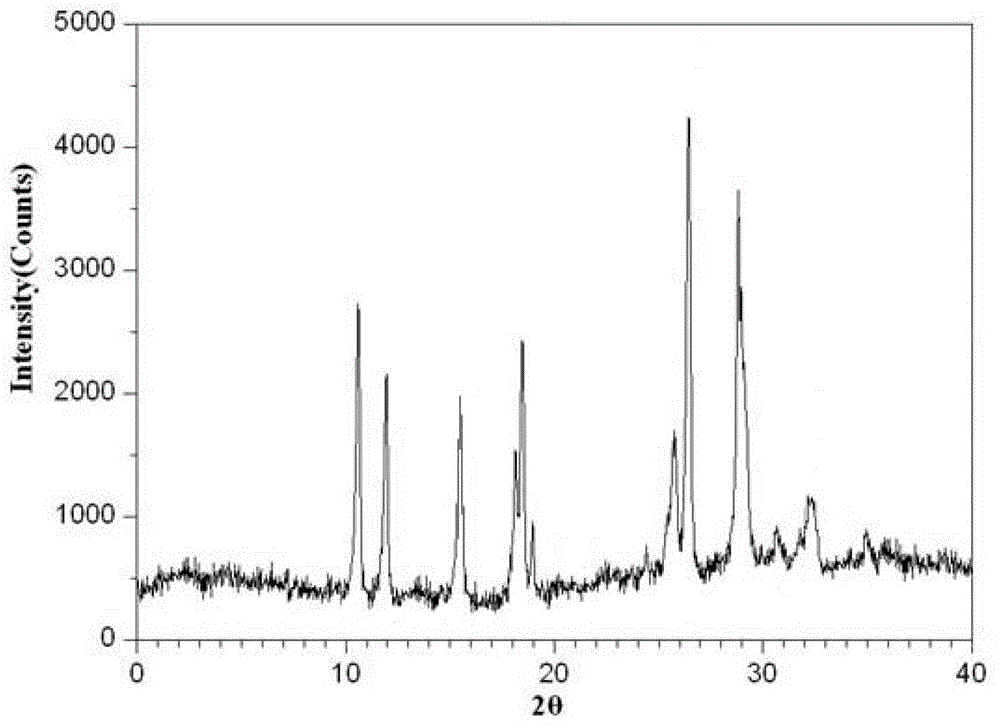
[0043] The preparation method comprises the steps of: taking cefminox sodium bulk drug, adding dimethyl sulfoxide, the volumetric dosage of dimethyl sulfoxide and the mass ratio of cefminox sodium being 7ml: 1g, stirring until completely dissolved, and adjusting the pH To 4.5, add activated carbon for decolorization, the amount of added activated carbon is 0.2% g / ml of the total volume of the medicinal solution, stir for 30min, filter to obtain a clear solution, move the clear solution into a pressure vessel, and control the pressure in the pressure vessel at Add diethyl ether dropwise under the condition of 1.5Mpa and stirring, the stirring speed is controlled at 25rmp, the amount of diethyl ether used is 3 times the volume of dimethyl sulfoxide, release the pressure after the dropwise addition, cool down to 0°C, let stand for 2h, filter and wash , and dried under reduced pressure to obtain white microcrystalline po...
Embodiment 2
[0046] The preparation method of cefminox sodium compound:
[0047] The preparation method comprises the steps of: taking cefminox sodium bulk drug, adding dimethyl sulfoxide, the volumetric dosage of dimethyl sulfoxide and the mass ratio of cefminox sodium being 10ml: 1g, stirring until completely dissolved, and adjusting the pH To 5.5, add activated carbon for decolorization, the amount of added activated carbon is 0.3% g / ml of the total volume of the medicinal solution, stir for 35min, filter to obtain a clear solution, move the clear solution into a pressure vessel, and control the pressure in the pressure vessel at Under the condition of 1Mpa and stirring, add diethyl ether dropwise, the stirring speed is controlled at 10rmp, the amount of diethyl ether used is 4 times the volume of dimethyl sulfoxide, release the pressure after the dropwise addition, lower the temperature to 5°C, let stand for 1h, filter, wash, Dry under reduced pressure to obtain white microcrystalline ...
Embodiment 3
[0050] Preparation of cefminox sodium sterile powder injection:
[0051] Weigh 99.5 g of cefminox sodium and 0.5 g of sodium benzoate prepared in Example 1 under aseptic conditions, place them in a solid powder mixer and mix them uniformly, and the raw materials obtained are transferred to a sterile preparation workshop for precision metering and subpackaging, each bottle Contains 0.25g of cefminox sodium, stoppered and capped, and the finished product is packaged for storage and sent for inspection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com