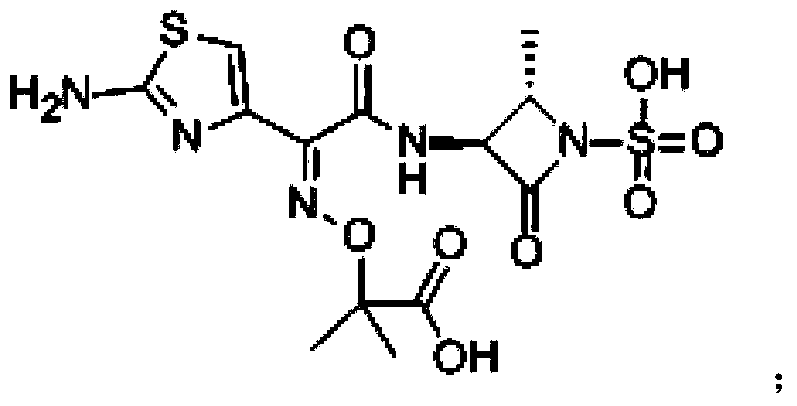
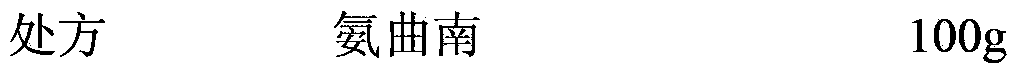Aztreonam compound, as well as preparation method and pharmaceutical composition thereof
An aztreonam and compound technology, applied in the field of medicine, can solve problems such as product contamination, achieve the effects of less impurity content, easy mixing, and improved drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
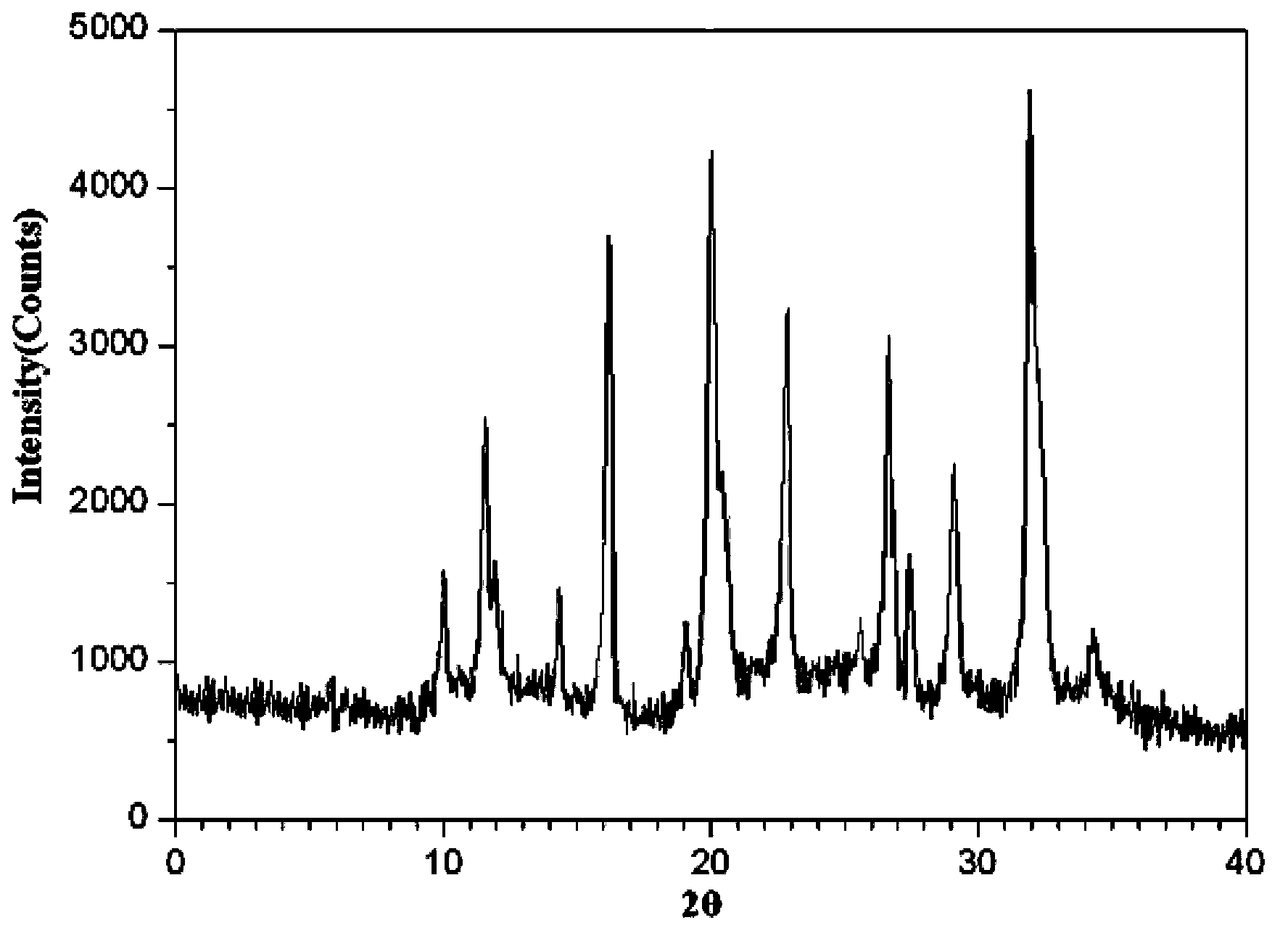
[0055] Preparation of aztreonam compound:
[0056] Dissolve 50 g of aztreonam crude drug completely in 150 ml of mixed solvent of dimethyl sulfoxide / acetone, the volume ratio of dimethyl sulfoxide to acetone is 5:1, and adjust the pH value of the liquid with triethylamine or acetic acid to 4.0, then add activated carbon, stir and adsorb for 30 minutes, filter for decarbonization and sterilization, add activated carbon in an amount of 0.2% g / ml of the total volume of the medicinal solution, and obtain a clear solution, move the clear solution into a pressure vessel, and put it in the pressure vessel Under the conditions of pressure of 1Mpa and temperature of the clear solution in the pressure vessel at 65°C, slowly add ethanol and stir, the volume ratio of ethanol to mixed solvent is 5:1, and the stirring speed is 27rpm when adding ethanol dropwise, resulting in a white precipitate. Filter, wash twice with ethanol and deionized water successively, and dry under reduced pressure...
Embodiment 2
[0059] Preparation of aztreonam compound:
[0060] Dissolve 50 g of aztreonam crude drug completely in 250 ml of a mixed solvent of dimethyl sulfoxide / acetone, the volume ratio of dimethyl sulfoxide to acetone is 7:1, and adjust the pH value of the liquid with triethylamine or acetic acid to 5.0, then add activated carbon, stir and adsorb for 30min, filter for decarburization and sterilization, add activated carbon in an amount of 0.3% g / ml of the total volume of the medicinal solution, and obtain a clear solution, move the clear solution into a pressure vessel, and The pressure is 1Mpa, the temperature of the clear solution in the pressure vessel is 75°C, slowly add ethanol and stir, the volume ratio of ethanol to mixed solvent is 8:1, and the stirring speed is 28rpm when ethanol is added dropwise, and a white precipitate is produced. Filter, wash twice with ethanol and deionized water successively, and dry under reduced pressure to obtain white microcrystalline powder. Yiel...
Embodiment 3
[0063] Aztreonam sterile powder injection
[0064] Weigh 100 g of aztreonam and 50 g of arginine prepared in Example 1 under aseptic conditions, place them in a solid powder mixer and mix them uniformly, and transfer the obtained raw materials to a sterile preparation workshop for precise metering and packaging. Each bottle contains ammonia Qunan 1.0g, stoppered and capped, the finished product is packaged for storage and sent for inspection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com