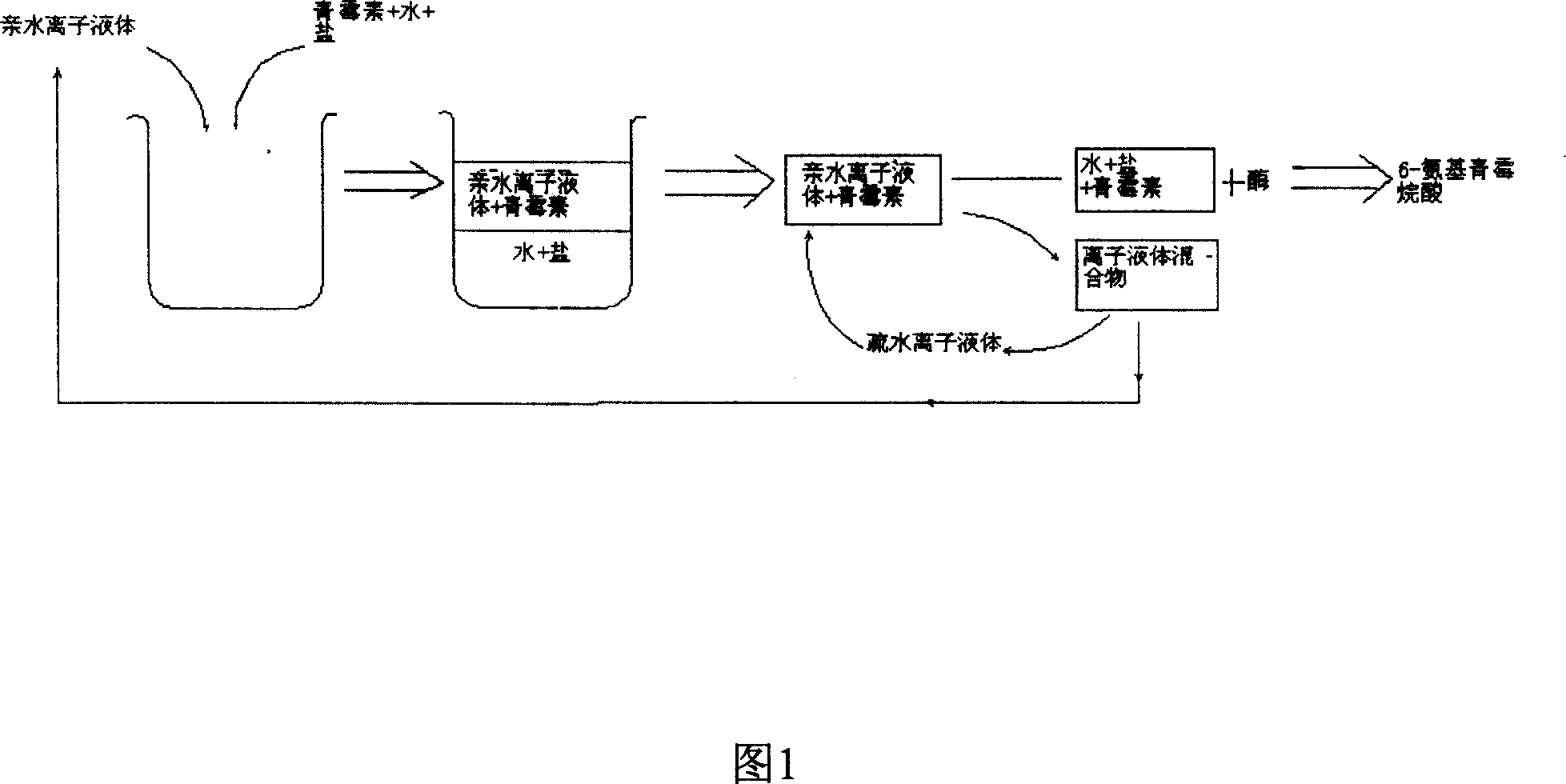
Method of preparing semisynthetic antibiotic 6-amino penicillanic acid by ion liquid extraction penicillin and enzymic catalytic reaction coupling
A technology of aminopenicillanic acid and ionic liquids, applied in organic chemistry, fermentation, etc., can solve the problems of reduced penicillin potency, waste of energy, and high content of ionic liquids, and achieve the effect of optimizing the extraction and catalytic process and realizing recycling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
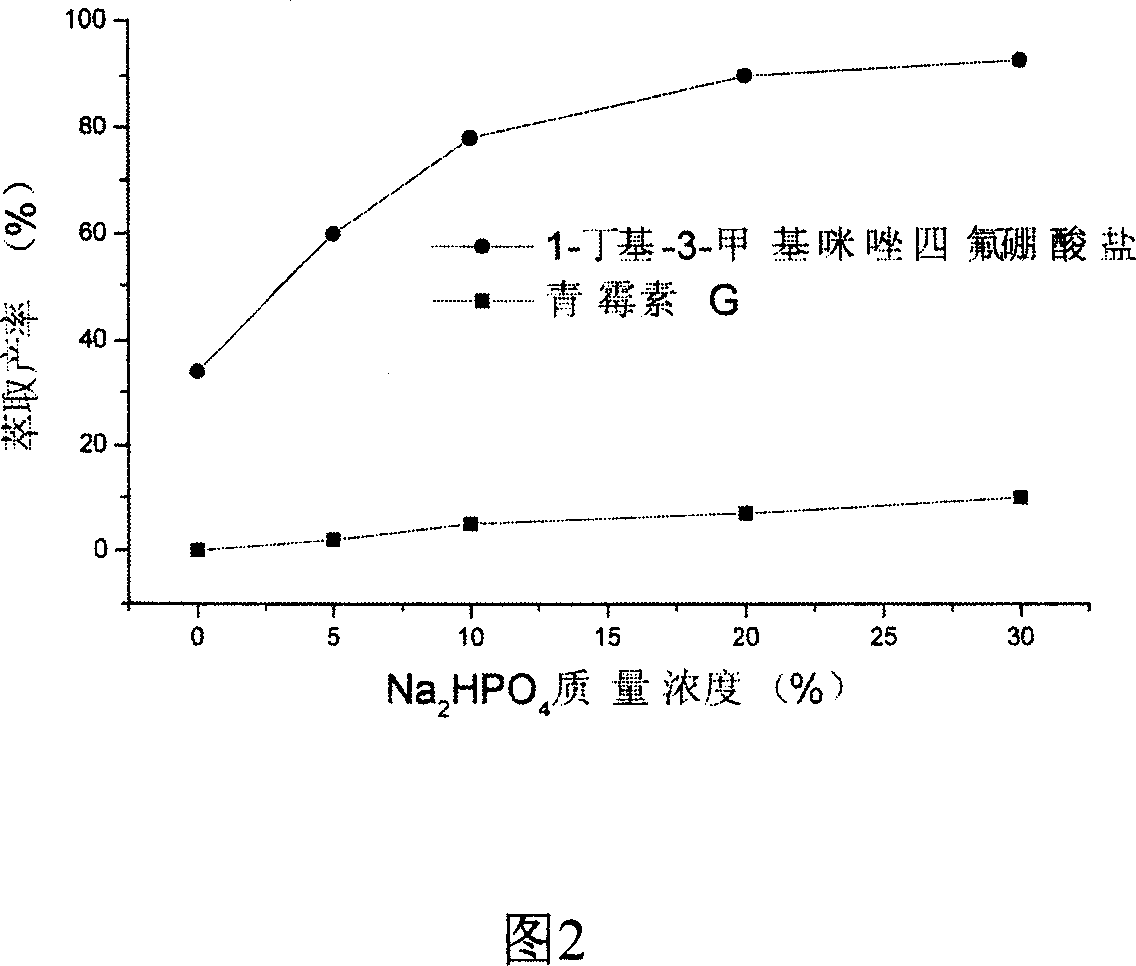
[0041] Get the ionic liquid light phase of 10ml two aqueous phases, contain 47% (w / w) [C 4 mim] BF 4 , 18% (w / w) Na 2 HPO 4 12H 2 O and penicillin 1.4%, add 2ml [C 4 mim]PF 6 , oscillated for 2 minutes, and allowed to stand for stratification. It can be found that the hydrophobic phase was 5.8ml, and 80% of [C 4 mim] BF 4 was extracted into the hydrophobic phase, but less than 4% of the penicillin was extracted. The volume of the upper phase aqueous solution is 6.2ml, containing 1.44% penicillin and a small amount of Na 2 HPO 4 . Take 5ml containing penicillin and Na 2 HPO 4 15 mg of immobilized penicillin acylase was added to the aqueous solution of the solution, reacted at 37° C., and 6-aminopenicillanic acid was isolated. The measured enzyme activity was 162 IU / g, which was 85% of the activity of 190 IU / g in pure water. Get 5ml ionic liquid extraction phase, directly adopt the pure water washing of 1: 1 ratio, about 52% [C 4 mim] BF 4 separated from the hydrop...
Embodiment 2
[0043] Get the ionic liquid light phase of 10ml two-phase aqueous phase, contain 58% (w / w) [C 4 mim] BF 4 , 9.3% (w / w) Na 2 HPO 4 12H 2 O and penicillin 1%, add 1ml [C 4 mim]PF 6 , shake for 2 minutes, stand to separate layers, the hydrophobic phase is 5.5ml, 78% [C 4 mim] BF 4 Extracted into the hydrophobic phase, 6% of the penicillin was extracted. The volume of the upper phase is 5.5ml, containing 1.6% penicillin and a small amount of Na 2 HPO 4 . Take 5ml containing penicillin and Na 2 HPO 4 15 mg of immobilized penicillin acylase was added to the raffinate, reacted at 37° C., and 6-aminopenicillanic acid was isolated. The measured enzyme activity was 145 IU / g, which was 76% of the activity of 190 IU / g in pure water. Get 5ml ionic liquid extractive phase, wash with pure water 70 ℃ of 1: 1 ratio, about 93% [C 4 mim] BF 4 separated from the hydrophobic phase.
Embodiment 3
[0045] Get the ionic liquid light phase of 10ml two-phase aqueous phase, contain 34.5% (w / w) [C 4 mim] BF 4 and 21.4% (w / w) Na 2 HPO 4 12H 2 O, and penicillin 0.8%, add 1ml [C 4 mim]PF 6 , shaking for 2min, standing for stratification, it can be found that the hydrophobic phase is 3.7ml, 82% [C 4 mim] BF 4 was extracted into the hydrophobic phase, but less than 8% of the penicillin was extracted. The volume of the upper phase is 7.5ml, containing 0.9% penicillin and a small amount of Na 2 HPO 4 . Take 5ml containing penicillin and Na 2 HPO 4 15 mg of immobilized penicillin acylase was added to the aqueous solution, reacted at 37°C, and 6-aminopenicillanic acid was isolated. The measured enzyme activity was 164 IU / g, which was 85% of the activity of 190 IU / g in pure water. Get 3.5ml ionic liquid extraction phase, directly adopt the pure water washing of 1: 1 ratio, about 56% [C 4 mim] BF 4 separated from the hydrophobic phase.
PUM
| Property | Measurement | Unit |
|---|---|---|
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com