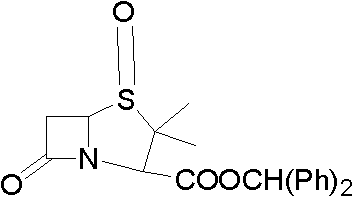
Method for preparing penicillanic acid sulfoxide diphenyl methyl ester
A technology of diphenylmethyl penicillanic acid sulfoxide and diphenylmethanol, which is applied in the field of preparation of diphenylmethyl penicillanic acid sulfoxide, and can solve problems such as high cost, complicated preparation process, and poor yield quality , to achieve the effect of quality improvement, good chemical selectivity, yield and quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
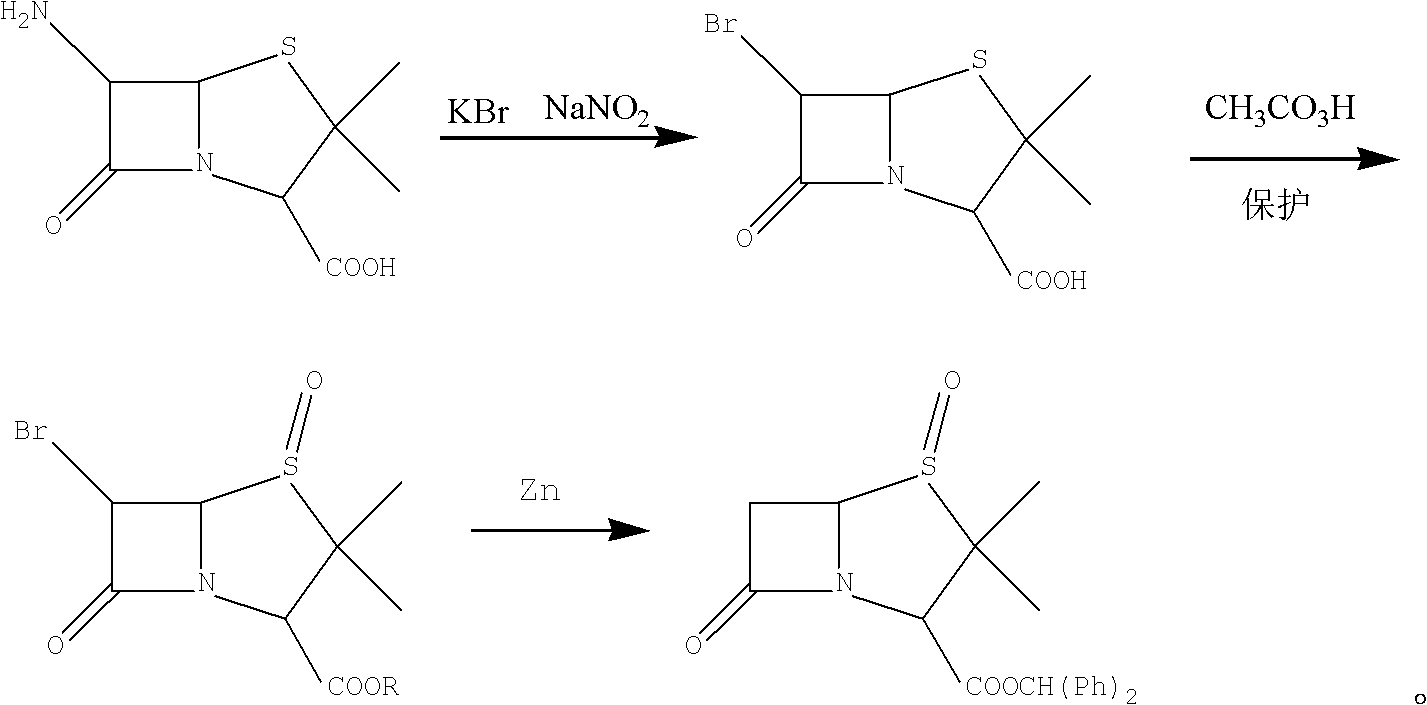
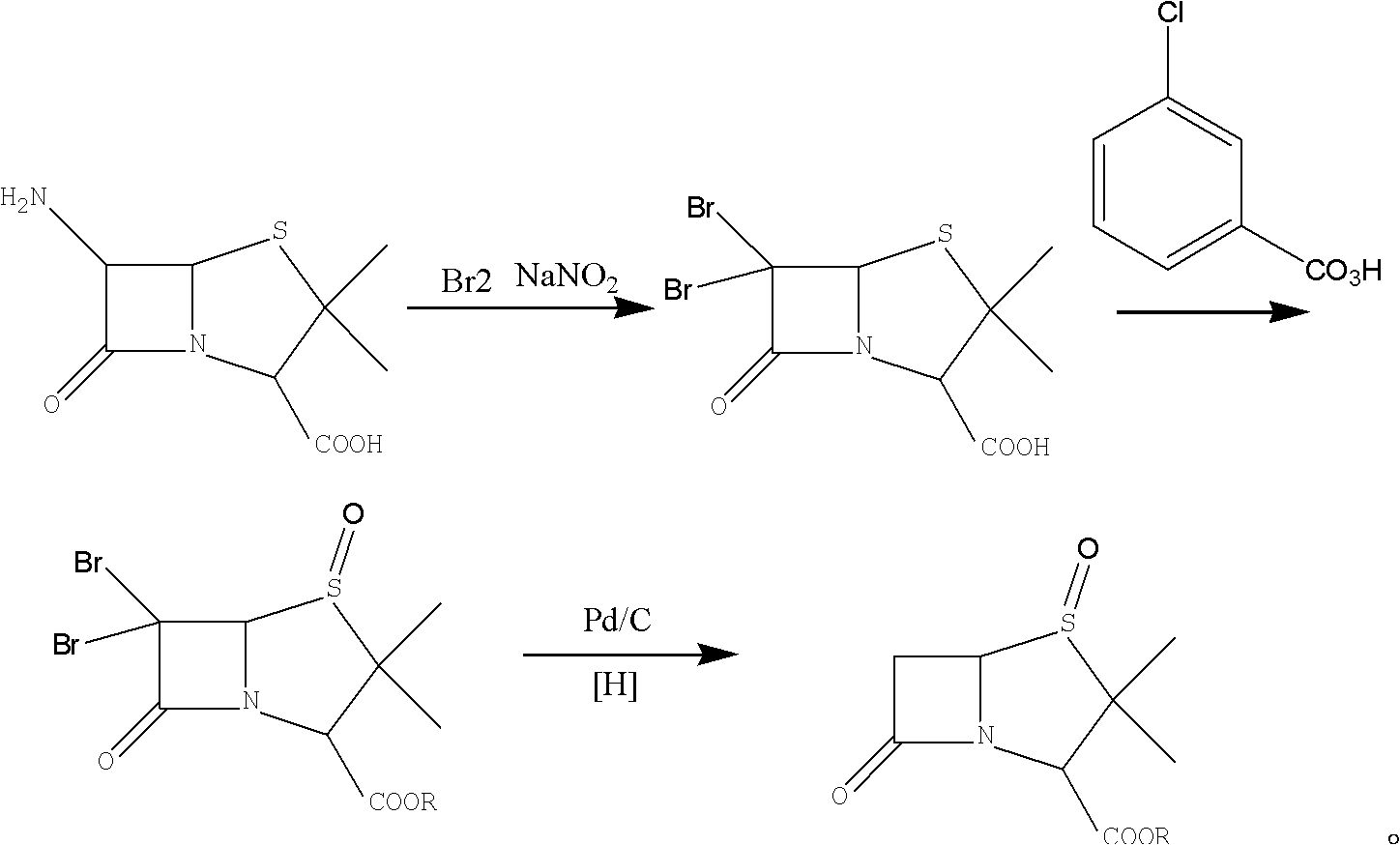
[0026] Embodiment 1, the preparation of 6-APA brominated penicillanic acid (compound iii):
[0027] Add 100g of 40% ethanol aqueous solution to a 500ml four-necked flask, add 50g, cool 40% hydrobromic acid to -5°C, add 45g of 6-APA, and dropwise add a solution made of 20g of sodium nitrite and 38g of water, and control the temperature at -5°C. ~0°C, about 1.5h, after the dropwise addition, keep warm at 0~5°C for 1.0h, add 180g CH2CL2 and stir for 10min after the heat preservation, stand still for 20min to separate layers, extract the water phase with 180gCH2Cl2 again, combine the organic phase, add 250ml of water, wash and stir After 10 minutes, the solution was separated for 20 minutes to obtain compound (iii) 6-APA bromide penicillanic acid dichloromethane solution. It was detected that 52.5 g of compound (iii) was present, and the molar yield was 91%.
[0028] Adopt KBr, Br in the past Differently as the brominating agent, the present invention adopts hydrobromic acid as th...
Embodiment 2
[0029] The preparation of embodiment 2,6-APA brominated penicillanic acid (compound iv):
[0030] Preparation of catalytic oxidation system:
[0031] Add 45g of 30% hydrogen peroxide and 1.5g of molybdenum acetylacetonate into a four-neck flask equipped with a stirrer, a thermometer, and warm it to 0-5°C. Adjust the pH to 2.0 with a 16% hydrochloric acid solution. , the color of the solution just disappears at this time.
[0032] Add the above prepared dichloromethane phase containing compound (iii) to the flask, and cool to -8°C. Add the above catalytic oxidation system solution, control the temperature at -8-2°C, complete the dropwise addition within 30 minutes, and keep warm at -5-0°C 2.0h, filter, the filter cake was washed with 20ml CH2Cl2, then washed with 10ml water, dried by centrifugation, and vacuum-dried at 20-25°C for 20h to obtain 50.0g of compound iv) with a molar yield of 90% and HPLC≥99%.
[0033] Compound (iii) has an unstable quaternary β-lactam ring struct...
Embodiment 3
[0035] Embodiment 3, the preparation of 6-APA bromide penicillanic acid benzhydryl sulfoxide (compound v):
[0036]Add 500ml of dichloromethane to the flask and cool to 0-5°C, then add 30.0g of compound IV, stir to dissolve and control the temperature at 0-5°C, add 20.0g of diphenylmethanol and 1.0g of DMAP, stir again to dissolve, and control the temperature 0~5℃, then dropwise add DCC.23.0g and 80ml of dichloromethane solution to control the temperature at 0~5℃, about 5.0h after dropping, keep warm for 1.0h, add 50ml of water to the filtered solution, wash, and stir at near temperature 0~5℃ for 10min Stand still for 20 minutes to separate layers, add 80ml of 8% sodium bicarbonate to the organic phase and stir for 10 minutes to stand still for 20 minutes to separate layers, distill the organic phase under normal pressure to recover dichloromethane, then evaporate to dryness under reduced pressure to obtain a white solid, then add 80g of toluene, ℃ for 1.0 h, cooled to -5 ℃ an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com