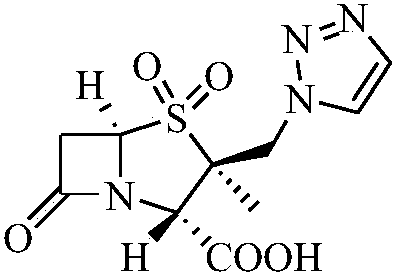
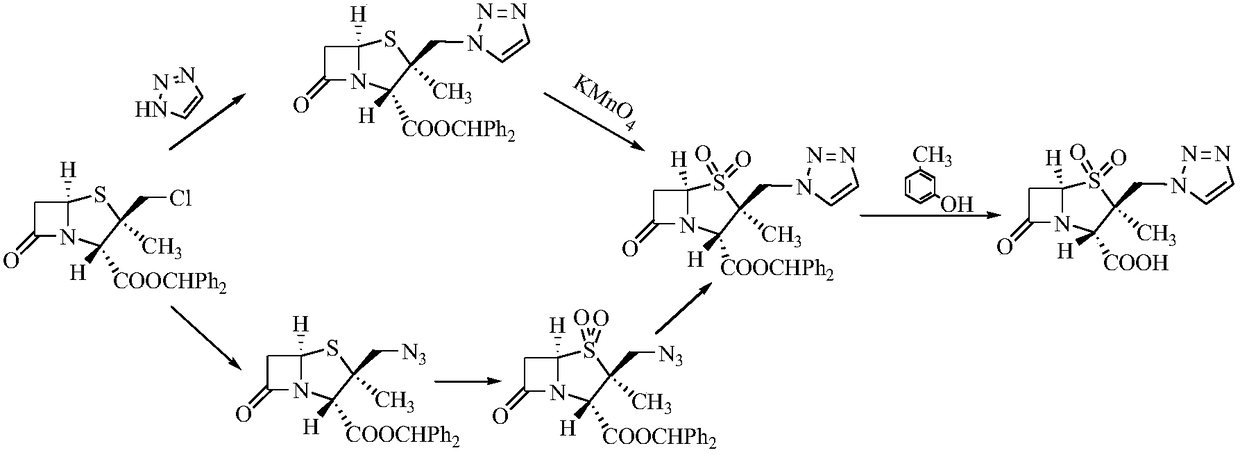
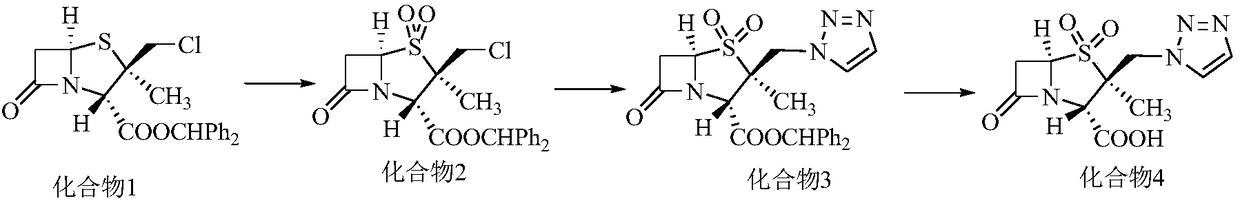
Preparation method of tazobactam
A technology of tazobactam and zobactam diphenylmethyl ester, which is applied in the field of preparation of tazobactam, can solve the problems of increased reaction steps, dangerous production process, long reaction time, etc., and achieves reduction of reaction steps and easy industrialization Production, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of 2β-chloromethylpenicillanic acid diphenylmethyl ester
[0032]
[0033] Add 110g of diphenylmethyl penicillane sulfoxide, 50g of 2-mercaptobenzothiazole, and 2000g of toluene into a 3L single-port bottle, heat up, reflux (divide water during reflux), start timing from 110°C, and reflux for 90-100 Minutes, cool down to 60-70°C, distill under reduced pressure, distill under reduced pressure until the feed liquid weight is 165g, stop distillation, cool down to 40-25°C, add 400g methylene chloride for subsequent use (thermal cracking feed liquid), in 3L five Add 120g of water, 300g of concentrated hydrochloric acid, and 500g of dichloromethane into the mouth bottle, cool down to -2~-6°C, and add the prepared thermal cracking feed solution and (21g of sodium nitrite + 150g of water) solution (dropping During the addition process, the temperature of the feed liquid is controlled to be -2~-6°C), after dripping, the temperature is controlled at -...
Embodiment 2
[0034] Embodiment 2: the preparation of double oxide
[0035] Add 500g of dichloromethane to the feed solution of compound 1 above, add 78g of potassium permanganate, cool down to 10-5°C, slowly add (50g of glacial acetic acid+370g of water+50g of industrial concentrated sulfuric acid) mixed solution, and control The temperature of the feed liquid is at 10-5°C, after the addition is completed, the temperature is controlled at 5-10°C and kept for 1-2 hours. After the heat preservation is completed, TLC detects that the basic reaction of the raw materials is complete. Slowly add 50% hydrogen peroxide dropwise until the reaction solution turns light yellow to off-white. After the drop is completed, stir for 20-30 minutes, separate the liquids, and add 500g of water and 32g of solid carbonic acid to the organic phase. The solution made of sodium hydrogen was stirred for 20-25 minutes, separated, and the organic phase was concentrated under reduced pressure at 40°C to obtain an oil...
Embodiment 3
[0036] Embodiment 3: the preparation of upper triazole i.e. tazobactam diphenylmethyl ester
[0037] In the oil after the reaction of Example 2, add 270g acetone, 320g water, 30g triazole, 90g anion resin, 20g crown ether 18-crown 6 and 20g potassium iodide, heat to reflux, keep warm for 5-8h, and keep warm. TLC detects that the basic reaction of the raw material is complete, add 650g of dichloromethane, stir for 10-15 minutes, filter, wash the filter cake with 650g of dichloromethane, and wash with 350g of water; combine the filtrate and washing liquid, add 1100g of water, stir for 10-15 minutes, and separate the liquid . The organic phase was washed by adding 500ml of purified water, and the organic phase was concentrated under reduced pressure at 40°C to obtain an oily substance, which was heated to reflux by adding 200ml of ethyl acetate, kept for 0.5h, cooled to crystallize, filtered to obtain 93.4g of off-white solid, and obtained in four steps. The yield is 69.8%, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com