The preparation method of latamoxef sodium intermediate
A technology for latamoxef sodium and intermediates, which is applied in the field of synthesis of latamoxef sodium intermediates, can solve the problems of increased uncontrollable reaction, unsuitability for industrial production, and high production costs, and achieve economic burden reduction and simplification of production equipment , The effect of material cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
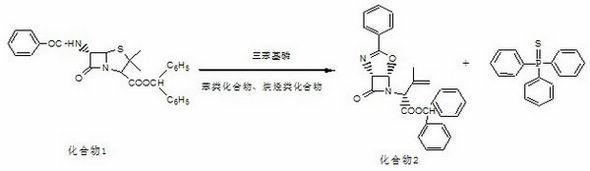
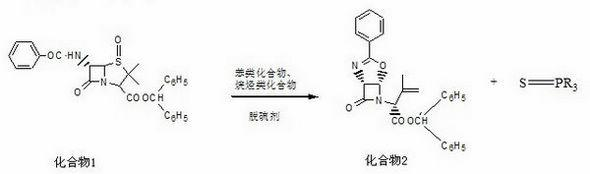
[0023] Example 1: Latamoxef sodium intermediate of the present invention is [1R-[1a,5a]]-3-methyl-2-(7-oxo-3-phenyl-4-oxa-2,6- The preparation method of diazabicyclo[3.2.0]hept-2-en-6-yl)-3-butenoic acid diphenylmethyl ester comprises the following processing steps:
[0024] a. Add 400mL of toluene, 30g of triphenylphosphine, 45g of 6-β-benzamide-4-oxo-penicillanic acid diphenylmethyl ester and 90mL of 1.2-diphenylmethyl ester in a dry and clean 1L four-necked bottle. For ethyl chloride, after setting up the reflux device, turn on the cooling water, control the internal temperature at 98-105°C, and make the material react under reflux;
[0025] b. When the residue of 6-beta-benzamide-4-oxo-penicillanic acid diphenylmethyl in the above reaction is ≤1.0%, the reaction ends, and then concentrated under reduced pressure at 50-60°C to evaporate to dryness, Then add 300mL of methanol and 280mL of acetonitrile mixed solvent to the evaporated concentrate, fully stir until dissolved a...
Embodiment 2
[0029] Embodiment 2: the difference between this embodiment and embodiment 1 is that
[0030] a. Add 380mL benzene, 32g triphenylphosphine, 45g 6-β-benzamide-4-oxo-penicillanic acid diphenylmethyl ester, 100mL dichloromethane to a dry and clean 1L four-neck bottle, and set aside After the reflux device, turn on the cooling water and control the internal temperature at 98-105°C to make the material react under reflux;
[0031] b. When the residue of 6-beta-benzamide-4-oxo-penicillanic acid diphenylmethyl in the above reaction is ≤1.0%, the reaction ends, and then concentrated under reduced pressure at 50-60°C to evaporate to dryness, Then add 300mL of ethanol and 280mL of acetonitrile mixed solvent to the evaporated concentrate, fully stir until dissolved and clarified, then lower the temperature to below 0°C and keep the crystal for 10 hours;
[0032] c, filter to remove the impurity triphenylthion that generates, then add 30mL ethanol solvent to wash the filter cake, then co...
Embodiment 3
[0035] Embodiment 3: the difference between this embodiment and embodiment 1 is,
[0036] a. Add 400mL benzene, 30g triphenylphosphine, 45g 6-β-benzamide-4-oxo-penicillanic acid diphenylmethyl ester, 100mL dichloromethane to a dry and clean 1L four-neck bottle, set aside Turn on the cooling water after the reflux device, control the internal temperature at 98-105°C, and make the material react under the reflux state;
[0037]b. When the residue of 6-beta-benzamide-4-oxo-penicillanic acid diphenylmethyl in the above reaction is ≤1.0%, the reaction ends, and then concentrated under reduced pressure at 50-60°C to evaporate to dryness, Then add 300mL of methanol and 280mL of acetonitrile mixed solvent to the evaporated concentrate, stir thoroughly until dissolved and clarified, then cool down to below 0°C to grow crystals for 8-10 hours;
[0038] c, filter to remove the impurity triphenylthion that generates, then add 30mL methanol to wash the filter cake, then combine the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com