Preparation method of 6-chloropenicillin sulfoxide diphenylmethyl ester and application thereof
A technology of diphenylmethyl sulfoxide and chloropenicillane, which is applied in the preparation and application field of 6-chloropenicillane diphenylmethyl sulfoxide, can solve the problems of low total yield, low safety, The three wastes are large, and the effects of improving the synthesis yield, high safety, and less waste water discharge are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Dissolve 40.0g of 6-APA (MW216.26, 0.185mol) in 100g of 30% hydrochloric acid, then add 20g of ethanol, then cool down to -5~5°C, after cooling down, slowly add 50g of 50% sodium nitrite aqueous solution (MW69.00, 0.347mol), after the dropwise addition, keep warm for 1-2 hours. After the heat preservation was completed, the reaction solution was extracted three times with 300 mL of dichloromethane, and the organic phases were combined to obtain a dichloromethane solution of AT-0, which was set aside.
[0060] Transfer the AT-0 dichloromethane solution to a 500mL three-necked flask, add 0.4g sodium tungstate (1%), cool down to 0-5°C, after cooling down, slowly add 40g 40% hydrogen peroxide (MW34.02, 0.471mol ), after dripping, keep warm for 3-4 hours, after keep warm, filter to get AT-1 wet product.
[0061] Add 150mL of dichloromethane and AT-1 wet product to a 500mL three-neck flask in sequence, cool down to 0-10°C, after cooling down, slowly add 10% ATMD dichlorometh...
Embodiment 2
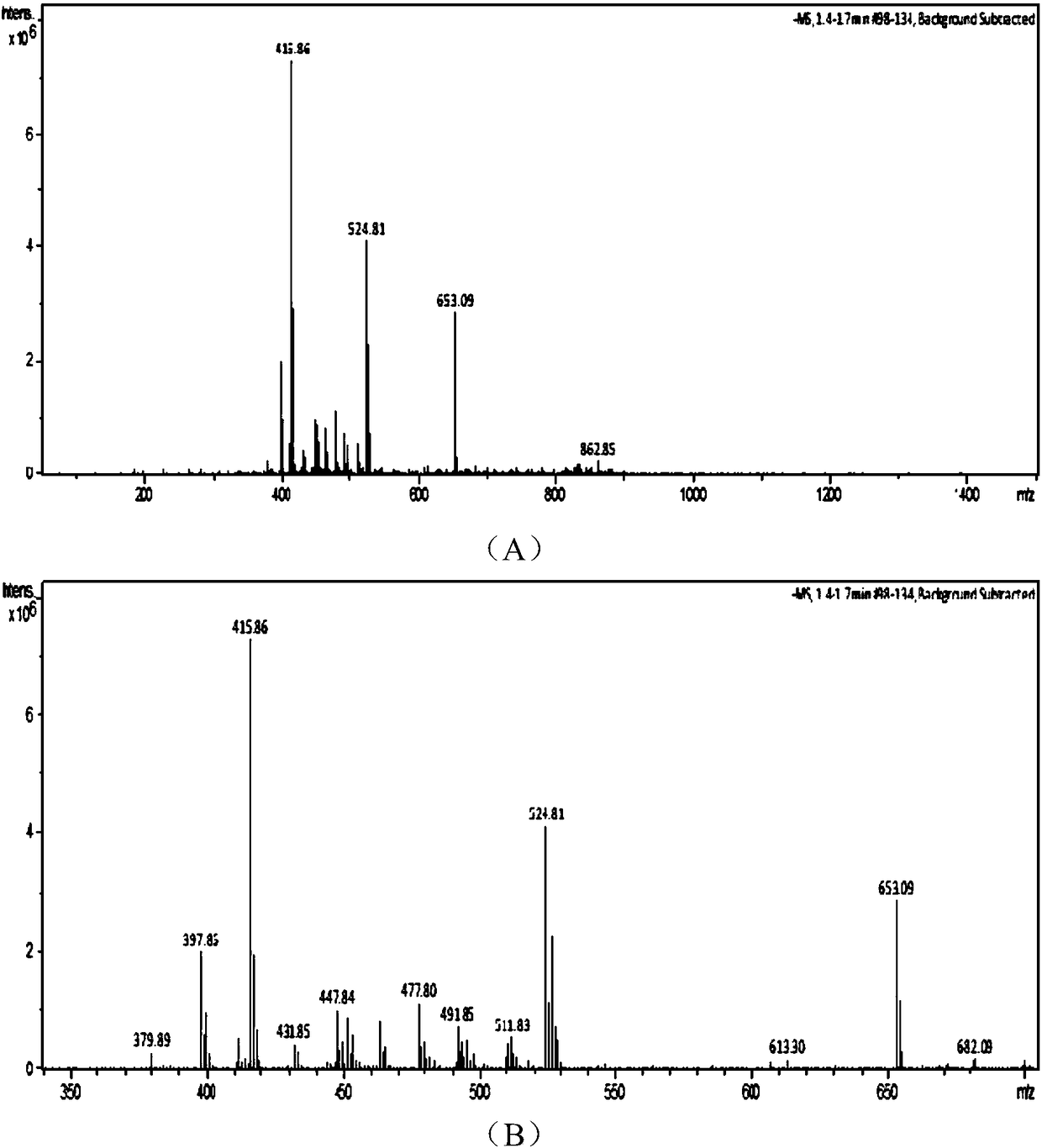
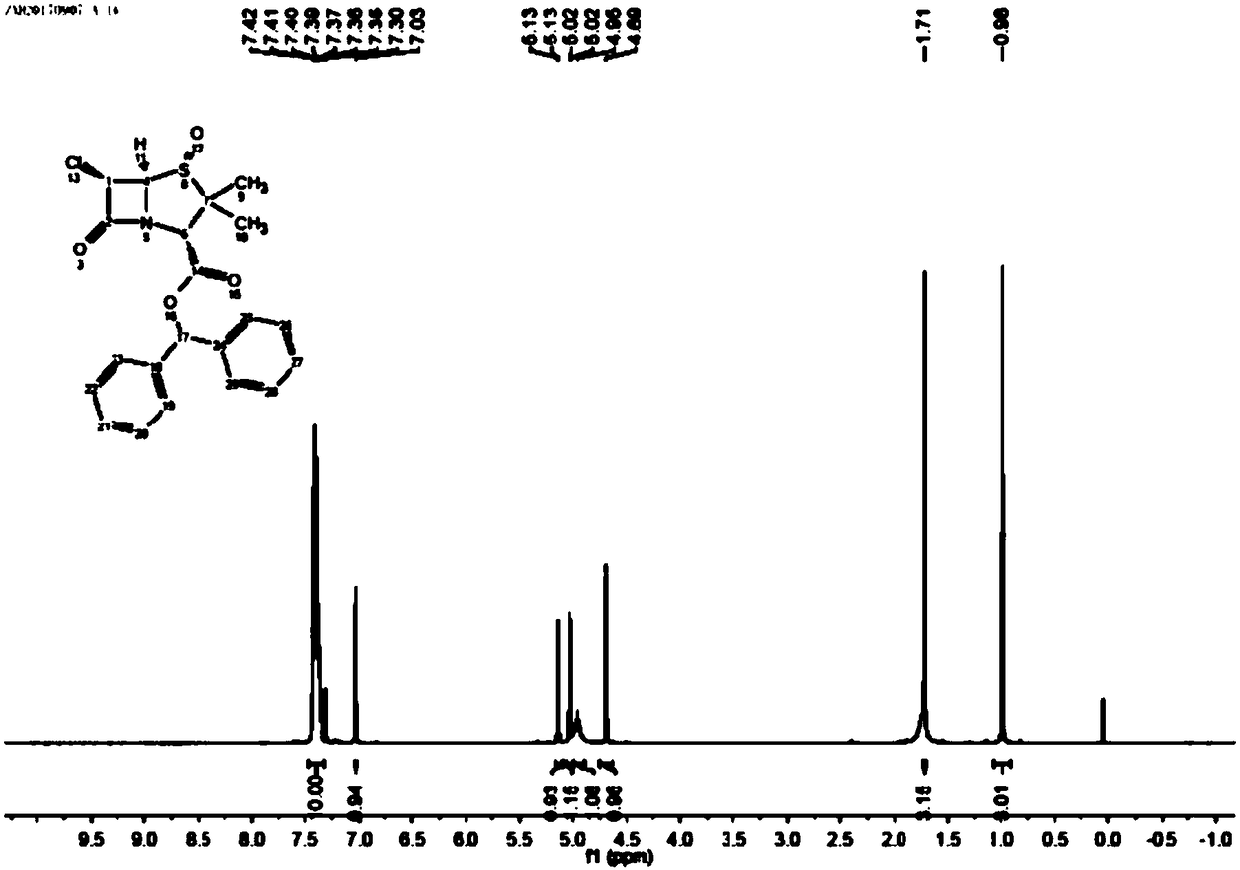
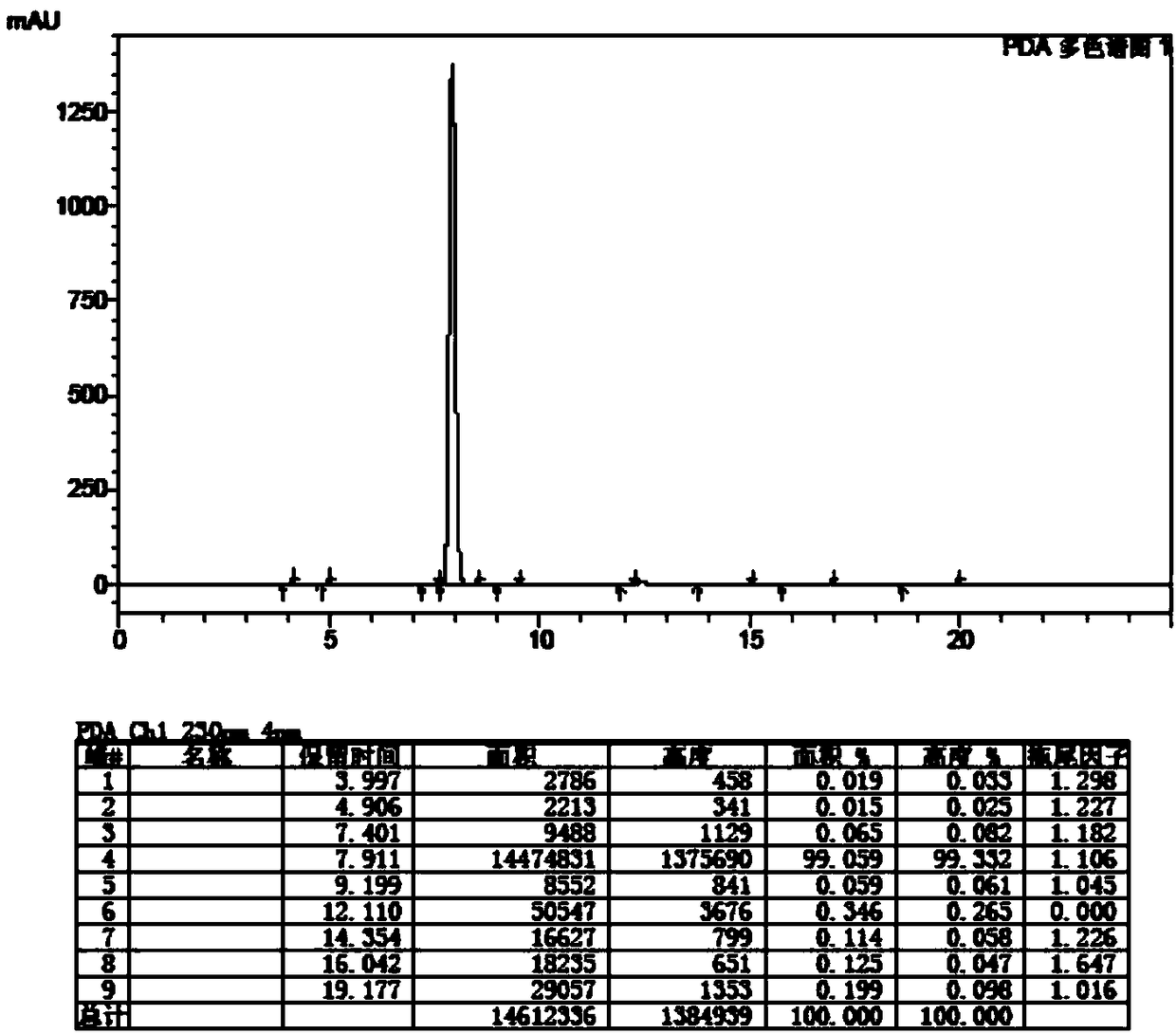
[0065] Add 250mL tetrahydrofuran and the AT-2 (MW417.91) wet product obtained in Example 1 to a 500mL three-necked flask, then add 136g of 36% ammonium acetate aqueous solution, cool down to 0-5°C, and slowly add 15g of Zinc powder, keep warm for 30 minutes, filter, let stand to separate layers, extract the water phase with 100mLTHF once, combine the organic phases, concentrate and crystallize under reduced pressure, filter to obtain the wet product of diphenylmethyl penicillane sulfoxide, at 50-60 After drying at ℃ for 8-12 hours, 58.2g of diphenylmethyl penicillane sulfoxide was discharged, the molar yield was 82.1% (based on 6-APA), the appearance was off-white crystalline powder, the content was 98.0%, the purity 99.0% (identified by HPLC, the retention time is consistent with the retention time of the standard substance, see the spectrogram image 3 ).
Embodiment 3
[0067] Dissolve 40.0g of 6-APA (MW216.26, 0.185mol) in 100g of 25% hydrochloric acid, add 20g of methanol, then cool down to -5~5°C, after cooling down, slowly add 40g of 50% sodium nitrite aqueous solution (MW69.00, 0.290mol), after the dropwise addition, keep warm for 1-2 hours.
[0068] After the heat preservation was completed, the reaction solution was extracted three times with 300 mL of ethyl acetate, and the organic phases were combined to obtain an ethyl acetate solution of AT-0, which was set aside.
[0069] Transfer the AT-0 ethyl acetate solution to a 500mL three-necked flask, add 0.4g sodium molybdate (1%), cool down to 0-5°C, after cooling down, slowly add 40g 30% hydrogen peroxide (MW34.02, 0.471mol ), after dripping, keep warm for 3-4 hours, after keep warm, filter to get AT-1 wet product.
[0070] Add 150mL of ethyl acetate and AT-1 wet product to a 500mL three-neck flask in turn, cool down to 0-10°C, after cooling down, slowly add 15% ATMD ethyl acetate solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com