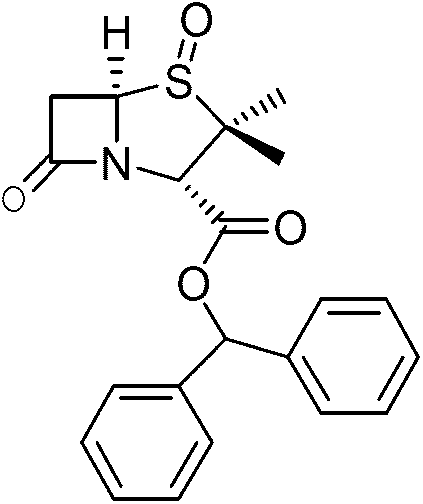
Preparation method of benzhydryl s-oxopenicillanate
A technology of diphenylmethyl sulfoxide and aminopenicillanic acid, applied in the direction of organic chemistry and the like, can solve the problems of complex process route, long synthesis period, poor product quality, etc., and achieves good reaction selectivity and short reaction period , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] Example 1: Preparation of penicillane sulfoxide acid (ⅳ)
[0039] Suck in 36% sulfuric acid (80kg) and 60L ethanol sequentially into the 500L reactor, and put in 45kg of 6-aminopenicillanic acid (ii). Temperature control -20°C~10°C Add 37% sulfuric acid (100kg) and 37% sodium nitrite aqueous solution (60kg) dropwise at the same time, then add 50% hypophosphorous acid aqueous solution (28kg) dropwise at temperature control -20°C~10°C, keep warm After 1-10 hours, extract with dichloromethane to obtain a dichloromethane solution of penicillanic acid (Ⅲ), with a purity of ≥90%. Add catalyst molybdenum acetylacetonate to the reaction kettle, and then add 50% hydrogen peroxide (45kg) dropwise at -15-10°C under temperature control. ), yield ≥ 90%, purity ≥ 95%.
example 2
[0040] Example 2: Preparation of benzhydryl penicillane sulfoxide (i)
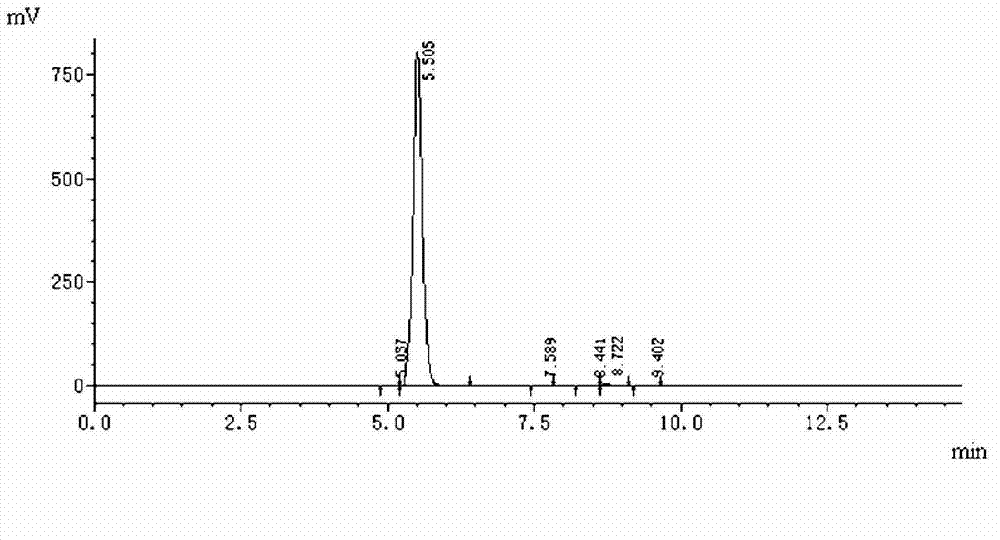
[0041] Pump acetone (36kg) and dichloromethane (265kg) into the 500L reactor, put in penicillane sulfoxide acid (ⅳ) (30kg), put in benzophenone hydrazone (39kg) and pump in 1 % KI in water (11 kg). Temperature control-20-0℃, add peracetic acid (75kg) dropwise, keep warm for 1~5h, wash the organic phase with 100ml brine and 100ml sodium bicarbonate solution, evaporate to dryness under reduced pressure, add toluene (50kg), temperature control -20 After stirring at ~15°C for 3 hours, centrifuge and dry to obtain diphenylmethyl penicillane sulfoxide (48kg), with a yield of ≥72% and a content of ≥98%. Purity chart see figure 1 , the content is 99.67% after comparison with the standard product verified by the enterprise.
[0042]
[0043]
example 3
[0044] Example 3: Preparation of penicillane sulfoxide acid (ⅳ)
[0045] Suck in 20% sulfuric acid (144kg) and 60L ethanol sequentially into the 500L reactor, and put in 45kg of 6-aminopenicillanic acid (ii). Control temperature -20℃~10℃, add 15% sulfuric acid (98kg) and 35% sodium nitrite aqueous solution (45kg) dropwise at the same time, then add 40% hypophosphorous acid aqueous solution (38kg) dropwise at temperature control -20℃~10℃, keep warm 1~10h, extract with dichloromethane to obtain penicillanic acid (Ⅲ) dichloromethane solution, purity ≥ 90%. Add catalyst molybdenum acetylacetonate to the reaction kettle, then add 50% hydrogen peroxide (45kg) dropwise at -15-10°C under temperature control, after the addition is complete, keep warm for 0.5-5h, and centrifuge to obtain penicillane sulfoxide acid (ⅳ) (37.8kg ), yield ≥ 90%, purity ≥ 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com