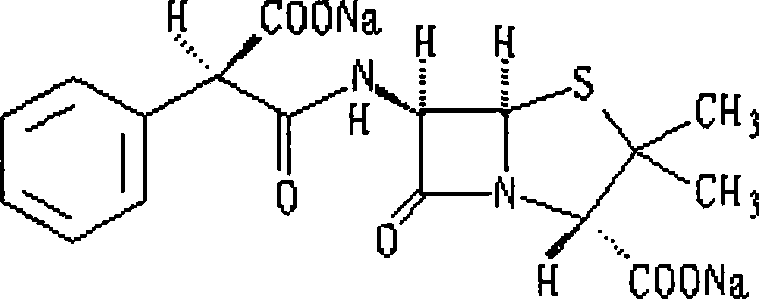
Method for synthesizing carbenicillin sodium
A technology of carbenicillin sodium and synthesis method, which is applied in the field of drug synthesis, can solve the problems of high cost and unsuitability for large-scale industrial production, and achieve the effects of convenient recovery and processing, reduction of production equipment, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In this embodiment, the specific operation steps are:
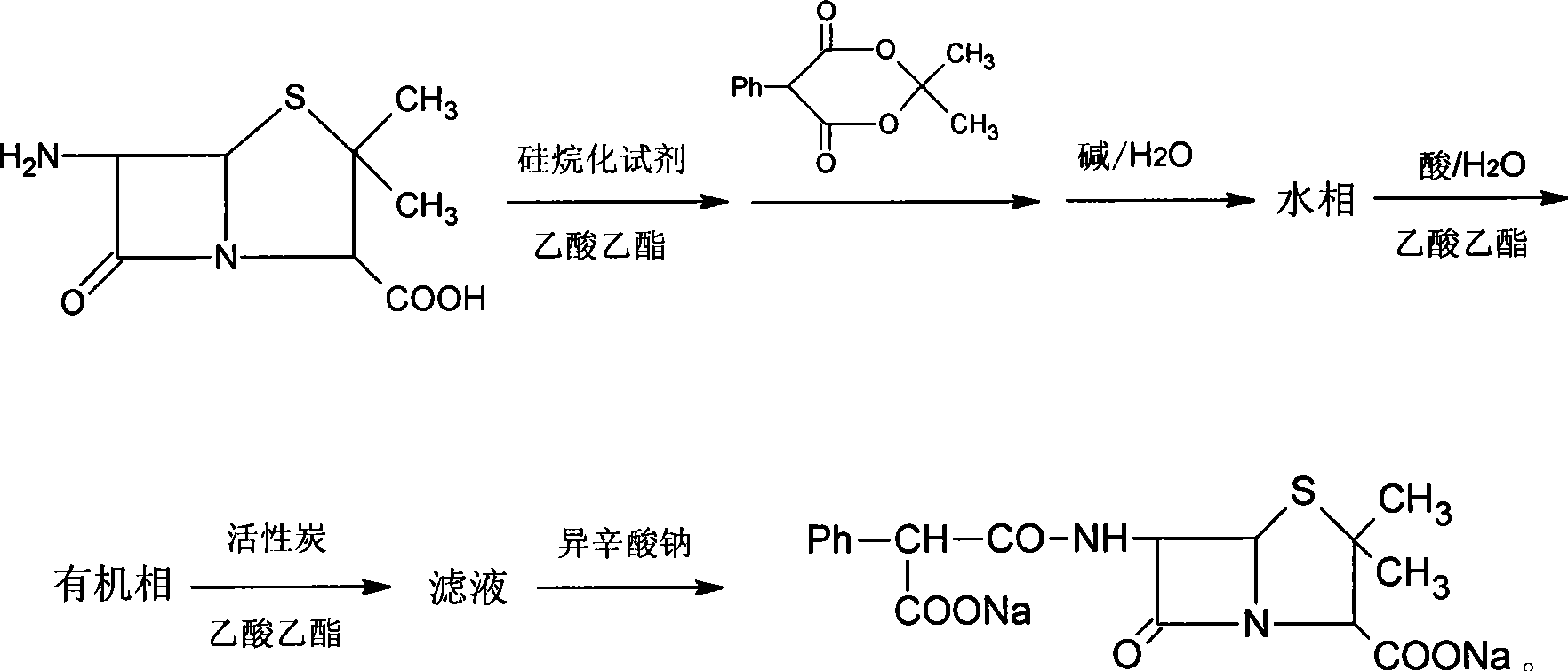
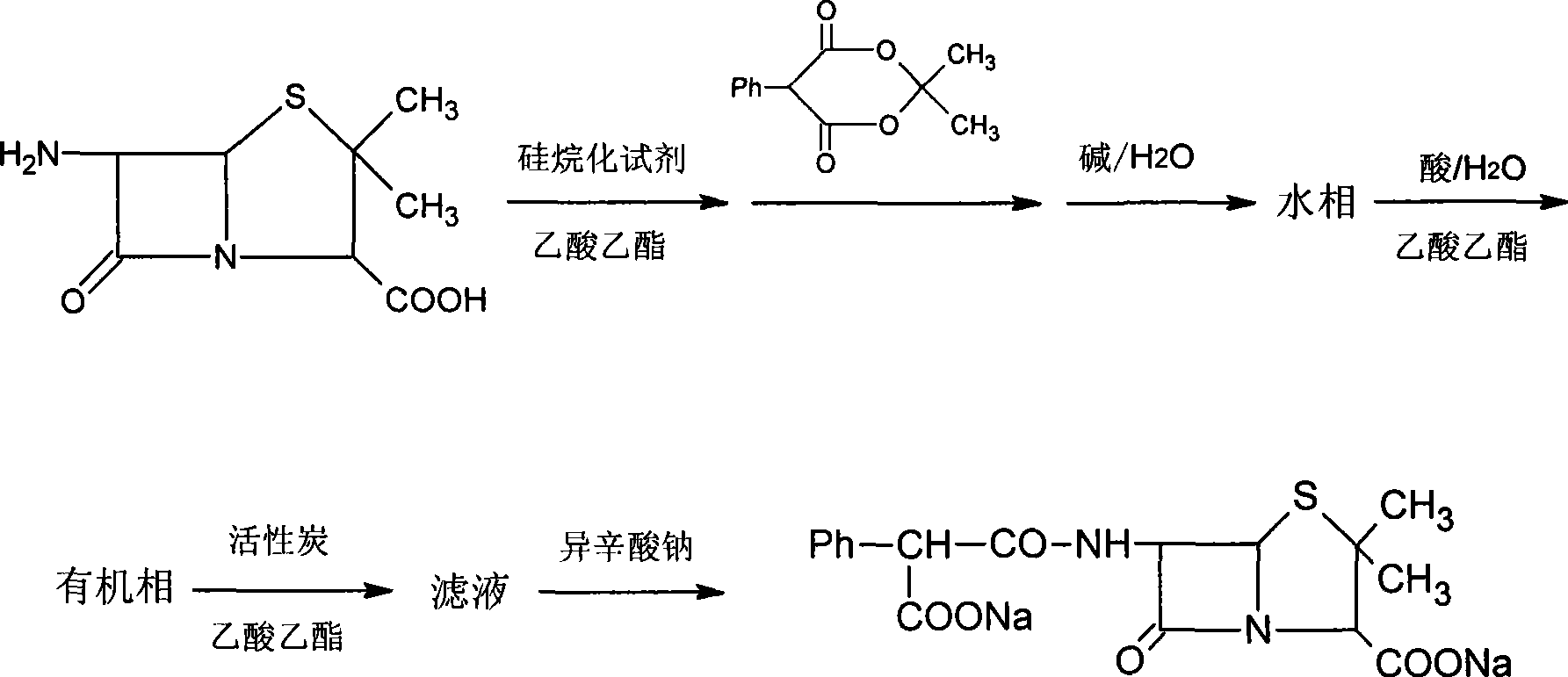
[0035] 1. Add 150ml of ethyl acetate and 20g of 6-aminopenicillanic acid into a 500ml three-necked bottle, stir, add 40g of N,N'-bistrimethylsilylurea, heat and reflux for 30min, cool to room temperature, and add while stirring Mesityl phenylmalonate 24g, stirred for 2.5 to 3 hours, until the reaction was complete (check the end point of the reaction with HPLC);
[0036] 2. Add 100ml of water to the reaction mixture, and adjust the pH to 6.5-7.5 with 30% sodium hydroxide solution while stirring.
[0037] 3. Stand still, after phase separation, remove the organic phase, take the water phase in a beaker, add 150ml of ethyl acetate under stirring conditions, adjust the pH to 1.0-1.5 with 10% HCl, stand still again, and after phase separation, take The organic phase.
[0038] 4. Add 60 ml of 25% sodium isooctanoate ethyl acetate solution to the organic phase, continue to stir for 1 hour after precipitation occurs, fi...
Embodiment 2
[0041] In this embodiment, the specific operation steps are:
[0042] 1. Add 150ml of ethyl acetate and 20g of 6-aminopenicillanic acid into a 500ml three-necked bottle, stir, add 44ml of hexamethyldisilazane, stir for 3h, then add 24g of mesityl phenyl malonate , and stirred for 2.5-3 hours until the reaction was complete (check the end of the reaction with HPLC).
[0043] 2. Add 100ml of water to the reaction mixture, and adjust the pH to 6.5-7.5 with 20% sodium carbonate solution while stirring.
[0044] 3. Stand still, carry out phase separation, remove the organic phase, take the water phase in a beaker, add 150ml of ethyl acetate under stirring conditions, adjust the pH to 1.0-1.5 with 10% sulfuric acid, let stand again, carry out phase separation, and take the organic phase .
[0045]4. Add 75 ml of 20% sodium isooctanoate in acetone to the organic phase while stirring, continue to stir for 1 hour after precipitation occurs, filter, and vacuum-dry the precipitate to o...
Embodiment 3
[0048] In this embodiment, the specific operation steps are:
[0049] 1. Add 150ml of ethyl acetate and 20g of 6-aminopenicillanic acid into a 500ml three-necked bottle, stir, add 50ml of N, O-bistrimethylsilylacetamide, stir for 2 hours, then add mesityl Ester 24g, stirred for 2.5 to 3 hours, until the completion of the reaction (check the end of the reaction with HPLC).
[0050] 2. Add 100ml of water to the reaction mixture, and adjust the pH to 6.5-7.5 with 8% sodium bicarbonate solution while stirring.
[0051] 3. Stand still, carry out phase separation, remove the organic phase, take the water phase in a beaker, add 150ml of ethyl acetate under stirring, adjust the pH to 1.0-1.5 with 20% phosphoric acid, let stand again, carry out phase separation, and take the organic phase.
[0052] 4. Add 150 ml of 10% sodium isooctanoate ethanol solution while stirring the filtrate, continue to stir for 1 h after precipitation occurs, filter, and vacuum-dry the precipitation to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com