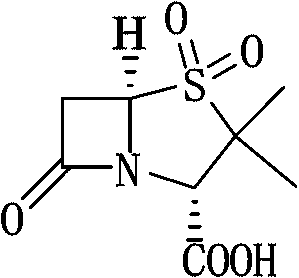
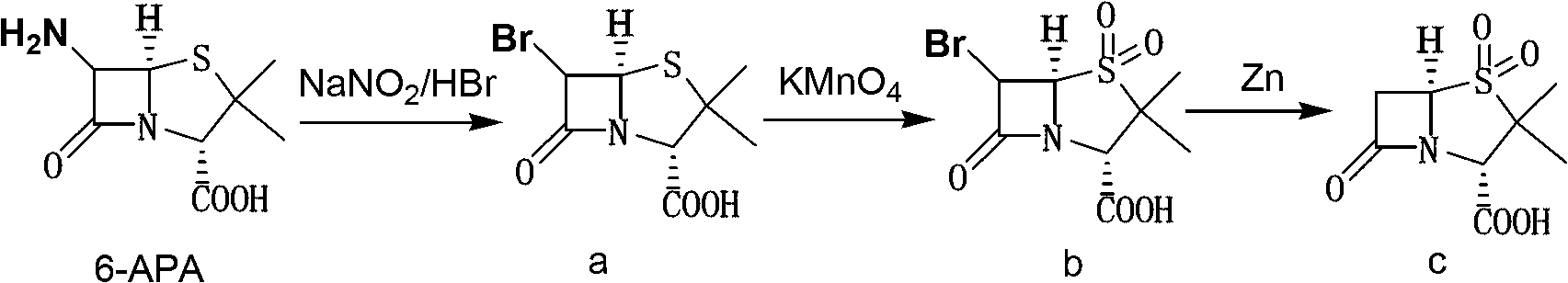
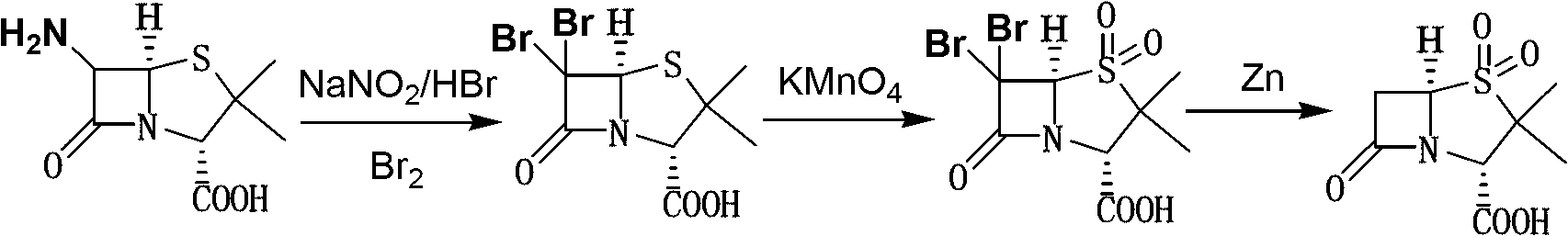Method for preparing sulbactam
A technology of aminopenicillic acid and addition amount, which is applied in the field of sulbactam preparation, can solve the problems of affecting product yield, large environmental pollution and high production cost, and achieves the effects of low cost, high yield and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of 6α-bromopenicillanic acid
[0028] Add 100ml of water and 110g of 48wt% hydrobromic acid (0.652mol) to the reaction bottle, start stirring, cool down to below 5°C, add 50ml of 95% ethanol, continue cooling to -15~-10°C, add 45g (0.208mol ) 6-APA, then dropwise add 25% sodium nitrite aqueous solution [25g sodium nitrite (0.362mol) + 38ml water], control the internal temperature between -10~0℃ during the dropping process, and finish adding in 3~4 hours After the addition, keep it warm at 0-5°C for 7 hours, add 130g of dichloromethane to extract once at the end of the heat preservation, extract the water phase with 130g of dichloromethane once, combine the organic phases, wash twice with 150ml saturated saline, wash After completion, add 250ml of water to the organic phase, control the temperature of the feed liquid at 0-5°C, slowly add solid sodium bicarbonate, adjust the pH value to 6-8, leave to separate and discard the organic phase to obta...
Embodiment 2
[0029] Embodiment 2: Preparation of 6α-bromopenicillane sulfonic acid
[0030] The 6α-bromopenicillanic acid aqueous solution prepared in Example 1 was continued to cool down to -5~0°C, and the phosphoric acid aqueous solution of potassium permanganate was added dropwise under stirring [49.3g potassium permanganate (0.312mol), 17ml Concentrated phosphoric acid, and 500ml of water], after adding, keep it at 0-5°C for 30 minutes; add 150ml of ethyl acetate, adjust the pH value to below 1.2 with 6N hydrochloric acid, control the temperature of the feed liquid at 0-5°C and add slowly 25% sodium bisulfite aqueous solution until the feed liquid is clarified, and the pH value of the feed liquid is controlled to be 1.25-1.35 during the dropping process. After the dripping, add 140g of sodium chloride solid and stir until dissolved, let stand for stratification, separate the organic phase, water The phase was extracted 3 times with 150ml×3 ethyl acetate, the organic phases were combine...
Embodiment 3
[0031] Embodiment 3: Preparation of sulbactam (penicillane sulfonic acid)
[0032] Add 500ml of water to the ethyl acetate feed liquid obtained in Example 2, lower the temperature to -5~0°C, control the temperature and start adding 61.2g of zinc powder (0.936mol) in batches, and at the same time add 6N hydrochloric acid dropwise to adjust the pH value of the feed liquid 3.5~4.0, keep warm for 40 minutes after adding zinc powder, add 200ml saturated sodium chloride aqueous solution, filter, add 6N hydrochloric acid dropwise to the filtrate to adjust the pH to 1.6-2.0, let it stand for stratification, and then use 150ml×3 Extract with ethyl acetate three times, combine the organic phases, distill to dryness under reduced pressure, add 500ml of distilled water, stir and raise the temperature to 40-45°C until the material liquid is clear, add 4.0g of activated carbon, keep it warm for 40 minutes, filter while it is hot, and cool the filtrate to 0~5℃, heat preservation and crystall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com