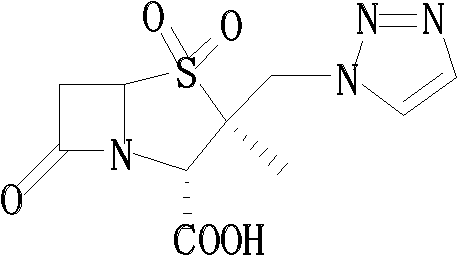
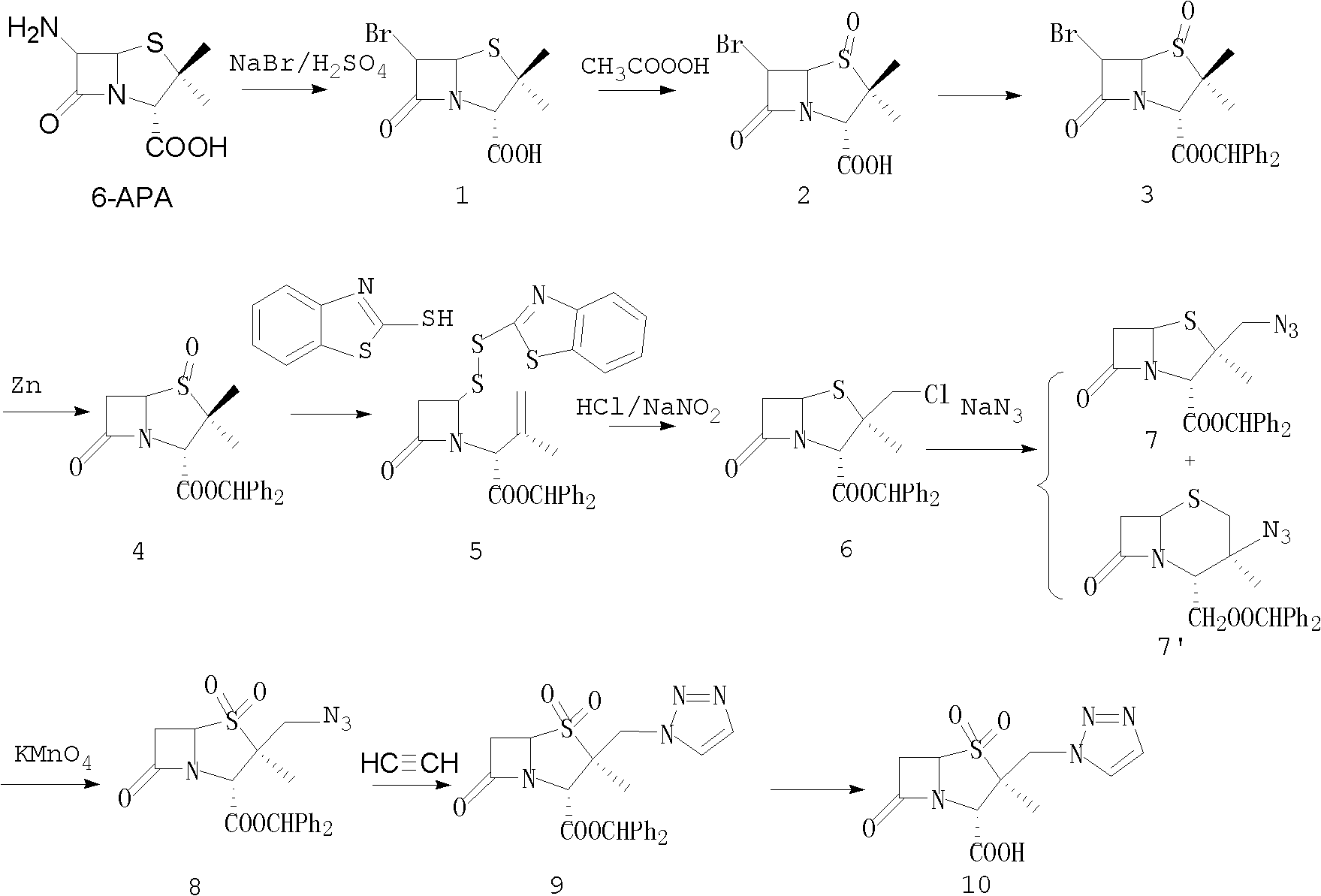
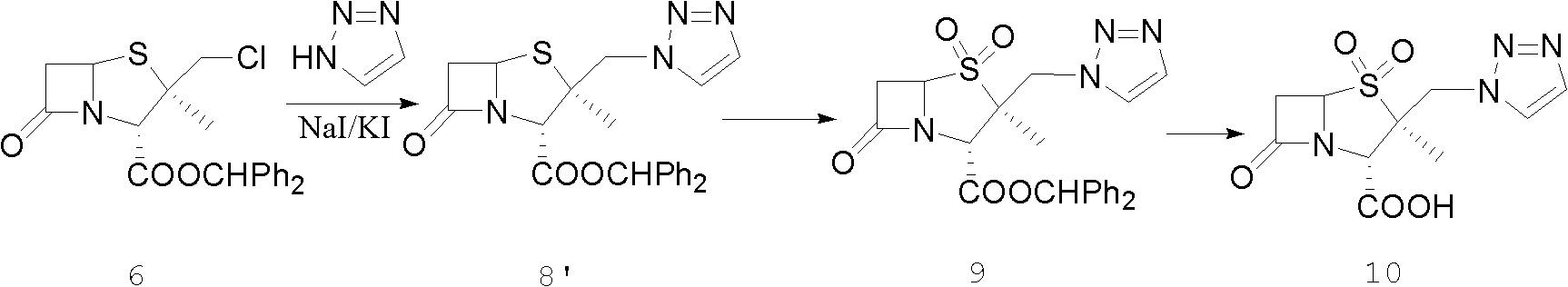
Tazobactam synthesis method
A technology of tazobactam and a synthesis method, which is applied in the field of synthesis of tazobactam, can solve the problems of lowering product yield and less stringent operation requirements, and achieves the effects of high yield and low impurity content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of benzhydryl β-chloromethyl penicillanic acid
[0037] With 6,6-dihydropenicillane sulfoxide benzhydryl as raw material, with reference to the preparation method of literature (Synthesis, 1986, (4), 292), obtain quantitative brownish yellow oil (according to 6,6-diphenyl Hydropenicillane sulfoxide benzhydryl 20g (52.2mmol) feeds intake, and 100% conversion rate calculates);
Embodiment 2
[0038] Embodiment 2: Preparation of 2β-chloromethylpenicillanic acid benzhydryl ester-1β-oxide (compound 6')
[0039] Add 240ml of dichloromethane to the oil obtained in Example 1, stir to dissolve, cool down to 0-5°C, start to slowly add 10.5g (67.8mmol) sodium perborate tetrahydrate in batches, and slowly heat up to 20°C after adding. Insulate at -25°C for 3-5 hours, slowly add 200ml of water, stir for 30 minutes, let stand to separate layers, distill the organic phase under reduced pressure to obtain an oily substance, separate by column chromatography, and obtain 2β-chloromethylpenicillanic acid diphenyl Methyl ester-1β-oxide, melting point: 55-60°C, ESI (m / z): 417; 1 HNMR (CDCl 3 )δ (ppm): 1.42 (3H, S), 3.04 (1H, dd), 3.25 (1H, dd), 3.52 (1H, d), 3.77 (1H, d), 4.25 (1H, m), 4.4 ( 1H, s), 6.53 (1H, s), 7.19 (4H, dd), 7.27 (4H, dd), 7.37 (2H, dd).
Embodiment 3
[0040] Example 3: Preparation of 2β-azidomethylpenicillanic acid diphenylmethyl ester-1β-oxide (compound 7”)
[0041]Add 240ml of N,N-dimethylformamide to the oil obtained in Example 2, stir to dissolve, keep the temperature at -5-5°C, add 5.08g (78.4mmol) sodium azide, and heat up to Incubate at 20-30°C for 20-30 hours, add 360ml of water, add 250ml of dichloromethane for extraction, let stand to separate layers, distill the organic phase under reduced pressure to obtain an oily substance, and separate it by column chromatography to obtain 2β-azidomethylpenicillane Acid benzhydryl-1β-oxide, melting point: 70-75°C, ESI (m / z): 426; 1 HNMR (CDCl 3 )δ (ppm): 1.41 (3H, S), 1.53 (1H, d), 1.74 (1H, d), 2.99 (1H, dd), 3.24 (1H, dd), 4.22 (1H, m), 4.35 ( 1H, s), 6.54 (1H, S), 7.19 (4H, dd), 7.27 (4H, dd), 7.37 (2H, dd).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com