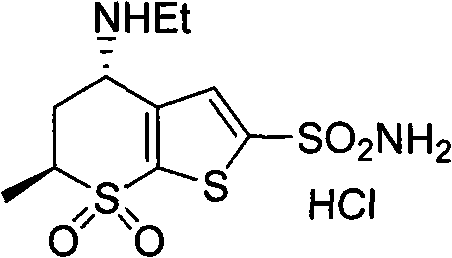
Method for synthesizing chiral dorzolamide hydrochloride
A synthesis method and compound technology, applied in the field of synthesis of chiral dulzolamide hydrochloride, can solve the problems of only 10% weight yield, low chiral resolution efficiency, easy racemization, etc., and achieve high optical purity , Avoid racemization, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
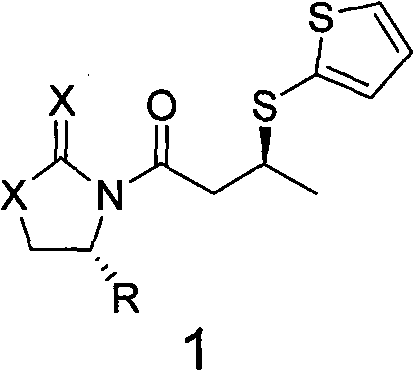
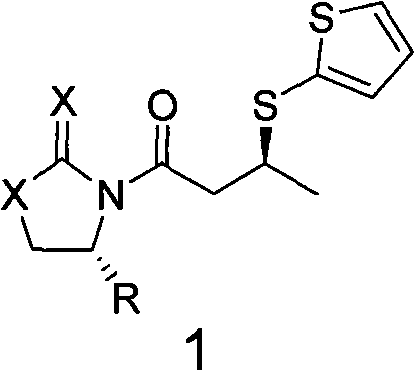
[0036] Synthesis of (R)-N-crotonyl-4-benzyl oxazolidinone (compound represented by general formula 2)
[0037] According to the literature Synlett. (2003, 2351), 177 g (1 mol) of chiral prosthetic oxazolidinone and 172 g (2 mol) of crotonic acid were dissolved in dichloromethane, and 412 g (2 mol) of dicyclohexylcarbonimide was added, The reaction was carried out at 25°C for 24 hours, followed by conventional post-treatment to obtain 240 g of solid, namely (R)-N-crotonyl-4-benzyloxazolidinone, which was directly used in the next reaction.
[0038]
[0039] C 14 h 15 NO 3 C 18 h 19 NO 3 S 2
[0040] Mol.Wt.: 245.27 Mol.Wt.: 361.48
[0041] In a 500ml dry four-neck flask, add 250ml of dichloromethane, 24.5g (0.1mol) of the solid (R)-N-crotonyl-4-benzyl oxazolidinone obtained above and 17.3g of titanium tetrachloride, and stir After dissolving, it was cooled to 0°C, and 20ml (0.13mol) of tetramethylethylenediamine and 12g (0.1mol) of 2-mercaptothiophene were...
Embodiment 2
[0043]
[0044] C 18 h 19 NO 3 S 2 C 8 h 8 OS 2
[0045] Mol.Wt.: 361.48 Mol.Wt.: 184.28
[0046] Dissolve the solid obtained in Example 1, that is, 29 g (0.08 mol) of the compound represented by general formula 1 in 300 ml of anhydrous tetrahydrofuran, add 50 ml of n-butyllithium (1.6M) dropwise at -78 ° C, and stir for 24 hours after the addition , adding ice water, conventional post-treatment to obtain 12g of solid product, i.e. the compound shown in general formula 3, its spectral data are as follows:
[0047] 1H NMR (500M, CDCl3) δ: 7.40(d, J=5.4Hz, 1H), 7.00(d, J=5.5Hz, 1H), 3.80(m, 1H), 2.89(dd, J=3.0, 16.5Hz , 1H), 2.65(dd, J=11.0, 16.4Hz, 1H), 1.50(d, J=7.0Hz, 3H).
Embodiment 3
[0049] Synthesis of (R)-N-crotonyl-4-isopropylthioxazolidinone (compound represented by general formula 2)
[0050] According to the document Synlett. (2003, 2351), 145g (1mol) of chiral prosthetic group-thioxazolidinone and 172g (2mol) of crotonic acid were dissolved in dichloromethane, and 412g (2mol) of dicyclohexylcarbonimide was added ), reacted at 25°C for 24 hours, and performed conventional post-treatment to obtain 200 g of solid, ie (R)-N-crotonyl-4-isopropylthiooxazolidinone, which was directly used in the next reaction.
[0051]
[0052] C 10 h 15 NO 2 S C 14 h 19 NO 2 S 3
[0053] Mol.Wt.: 213.30 Mol.Wt.: 329.50
[0054] In a 500ml dry four-necked flask, add 100ml of dichloromethane, 22.0g (0.1mol) of the solid (R)-N-crotonyl-4-isopropylthiooxazolidinone obtained above and trifluoromethanesulfonic acid 2.2g of titanium was stirred and dissolved and then cooled to -78°C, and 167ml (1mol) of tetramethylpropylenediamine and 24g (0.2mol) of 2-mercaptothiophen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com