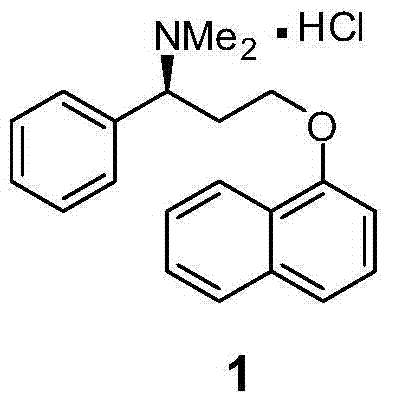
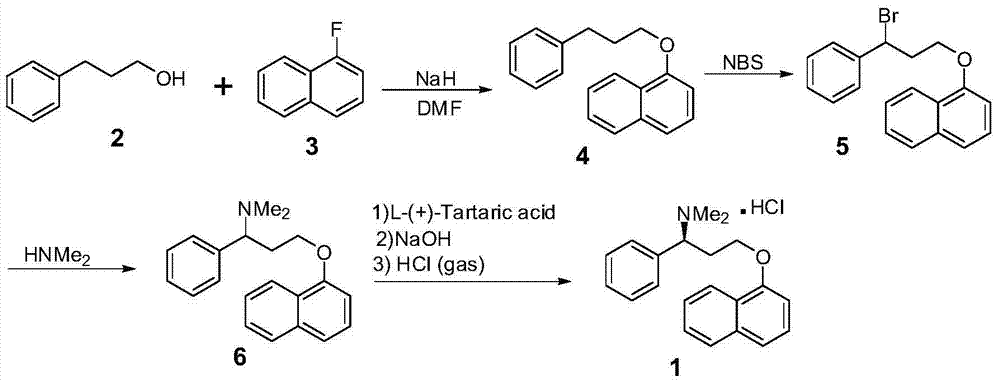
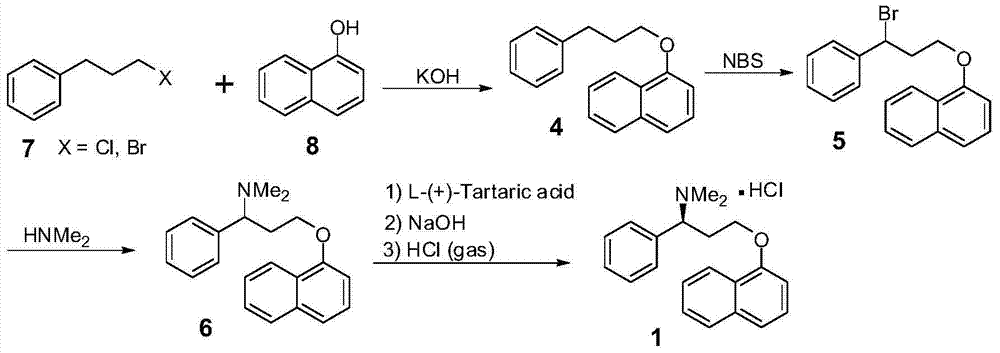
Dapoxetine hydrochloride synthetic method
A technique for the synthesis of dapoxetine hydrochloride, which is applied in the field of preparation of small-molecule chemical drugs, can solve the problems of low asymmetric reduction yield, reduced optical purity, and low total yield, so as to improve the optical purity of the product and the process The effect of shortening the route and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthetic compound(12)
[0036]
[0037] Compound (9) (16.8g, 0.1mol) was dissolved in 1,4-dioxane (40mL), and (-) diisopinepinyl chloride borane [(-)-DIPCl] was added at 25°C (64g, 0.12mol, 1.2eq, 60% tetrahydrofuran solution), at this temperature, reacted for 12h. Ice water (200 mL) was added slowly, keeping the temperature not exceeding 25°C, stirred for 30 minutes, and the layers were separated, and the aqueous phase was extracted with ethyl acetate (3×100 mL). The organic phases were combined, washed with saturated aqueous sodium bicarbonate to near neutrality, dried over anhydrous MgSO4, filtered, concentrated and evaporated to remove the organic solvent to obtain compound (12) (white solid, mp=56-58 ° C, yield 92%, HPLC purity 99.2 %, ee value = 99.2%).
[0038] 1 H-NMR (CDCl 3, 400MHz, δppm): 2.00(s,1H,OH),2.05-2.10(m,1H),2.12-2.26(m,1H),3.52-3.60(m,1H),3.72-3.80(m,1H) , 5.00 (dd, J=4.8Hz, J=8.2Hz, 1H), 7.33-7.40 (m, 5H).
Embodiment 2
[0040] Compound (9) (16.8g, 0.1mol) was dissolved in 2-methyltetrahydrofuran (60mL), and (-)-DIPCl (80g, 0.15mol, 1.5eq, 60% n-hexane solution) was added at 10°C, At this temperature, the reaction was 14h. Add ice water (250 mL) slowly, keep the temperature not exceeding 10°C, stir for 50 minutes, let stand to separate the layers, and extract the aqueous phase with ethyl acetate (3×100 mL). Combine the organic phases, wash with saturated aqueous sodium bicarbonate to nearly neutral, anhydrous MgSO 4 Dry, filter, concentrate and evaporate the organic solvent to obtain compound (12) (white solid, yield 95%, HPLC purity 99.5%, ee value=99.1%).
Embodiment 3
[0042] Compound (9) (16.8g, 0.1mol) was dissolved in THF (100mL), and (-)-DIPCl (106g, 0.20mol, 2.0eq, 60% n-heptane solution) was added at 40°C. Next, react for 16h. Add ice water (300 mL) slowly, keep the temperature not exceeding 0° C., stir for 50 minutes, stand to separate the layers, and extract the aqueous phase with ethyl acetate (3×100 mL). Combine the organic phases, wash with saturated aqueous sodium bicarbonate to nearly neutral, anhydrous MgSO 4 Dry, filter, concentrate and evaporate the organic solvent to obtain compound 12 (white solid, yield 96%, HPLC purity 99.3%, ee value=99.2%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com