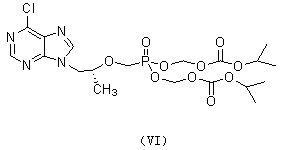
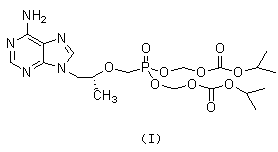
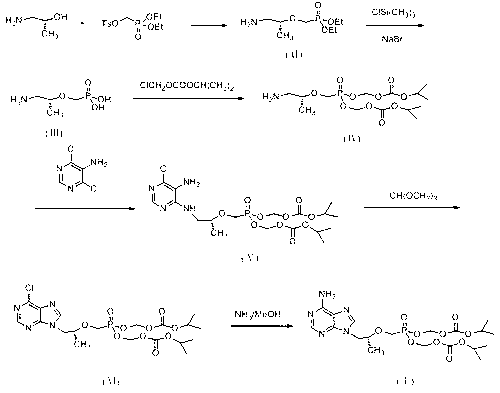
New process for synthesizing tenofovir disoproxil fumarate
A technology of tenofovir disoproxil and a new process, which is applied in the field of synthetic process of pharmaceutical compounds, can solve problems such as high price, dangerous use, product racemization, etc., and achieve safe production process, mild reaction conditions, and avoid product racemization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of (R)-2-O-(diethoxy-phosphono-methyl)-1-amino-2-propanol (II)
[0067] Add 30g (0.4mol) of (R)-(-)-1-amino-2-propanol and 300ml of DMF into a 500ml reaction flask, stir mechanically, add 136.4g (0.8mol) of magnesium tert-butoxide under stirring, and heat up to 70~80°C, slowly add 193.2g (0.6mol) of diethyl p-toluenesulfonyloxymethylphosphonate dropwise, after the dropwise addition, keep warm at 70~80°C for 6 hours, stop the reaction, and the reaction solution drops to room temperature , 100ml of acetic acid was added dropwise to neutralize the reaction solution, the reaction solution was distilled under high vacuum to remove the solvent DMF, the residual solution was added with 400ml of ethyl acetate and 200ml of water, stirred for 30 minutes, left to stand for layers, the water layer was extracted with 200ml of ethyl acetate, combined The ethyl acetate layer was washed successively with 300 ml of ice water and 300 ml of saturated brine, dried ov...
Embodiment 2
[0068] Example 2: Preparation of (R)-2-O-(diethoxy-phosphono-methyl)-1-amino-2-propanol (II)
[0069] Add 30g (0.4mol) of (R)-(-)-1-amino-2-propanol and 300ml of DMF into a 500ml reaction flask, stir mechanically, add 67.2g (0.6mol) of potassium tert-butoxide under stirring, and heat up to 70~80°C, slowly add 193.2g (0.6mol) of diethyl p-toluenesulfonyloxymethylphosphonate dropwise, after the dropwise addition, keep warm at 70~80°C for 6 hours, stop the reaction, and the reaction solution drops to room temperature , 40ml of acetic acid was added dropwise to neutralize the reaction solution, the reaction solution was distilled under high vacuum to remove the solvent DMF, the residue was added with 400ml of ethyl acetate and 200ml of water, stirred for 30 minutes, left to stand for layers, the water layer was extracted with 200ml of ethyl acetate, combined The ethyl acetate layer was washed successively with 300 ml of ice water and 300 ml of saturated brine, dried over anhydrous...
Embodiment 3
[0070] Example 3: Preparation of (R)-2-O-(dihydroxy-phosphono-methyl)-1-amino-2-propanol (Ⅲ)
[0071] Add 56.7g (0.252mol) of the compound of formula (II), 560ml DMF, and 91g (0.882mol) of sodium bromide into a 1L reaction flask, stir, and slowly add 137g (1.26mol) of trimethylchlorosilane dropwise under ice bath, and dropwise add After completion, the temperature was raised to 60°C to react for 16 hours. After the reaction was completed, the reaction solution was cooled to room temperature, filtered, and the filtrate was distilled off under high vacuum to remove the solvent DMF. Added 300ml of water and 300ml of ethyl acetate to the residue, stirred for 0.5 hours, and allowed to stand for stratification. Wash the water layer once more with 300ml of ethyl acetate, lower the temperature of the obtained water layer to 3~6°C, adjust the pH to 3~4 with 40% sodium hydroxide, and crystallize, keep warm at 5~8°C, stir and crystallize for 2 hours, filter , the filter cake was vacuum-d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com