High-molecular weight long-chain branched crystalline polylactic acid material and preparation method thereof
A polylactic acid material and long-chain branching technology, applied in the field of biodegradable polymer materials and its preparation, can solve the problem of low reactivity between hydroxyl and epoxy groups, unsuitability for large-scale production, and expensive bisoxazolines, etc. problems, to achieve excellent mechanical properties, environmentally friendly preparation process, good blow molding and foaming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
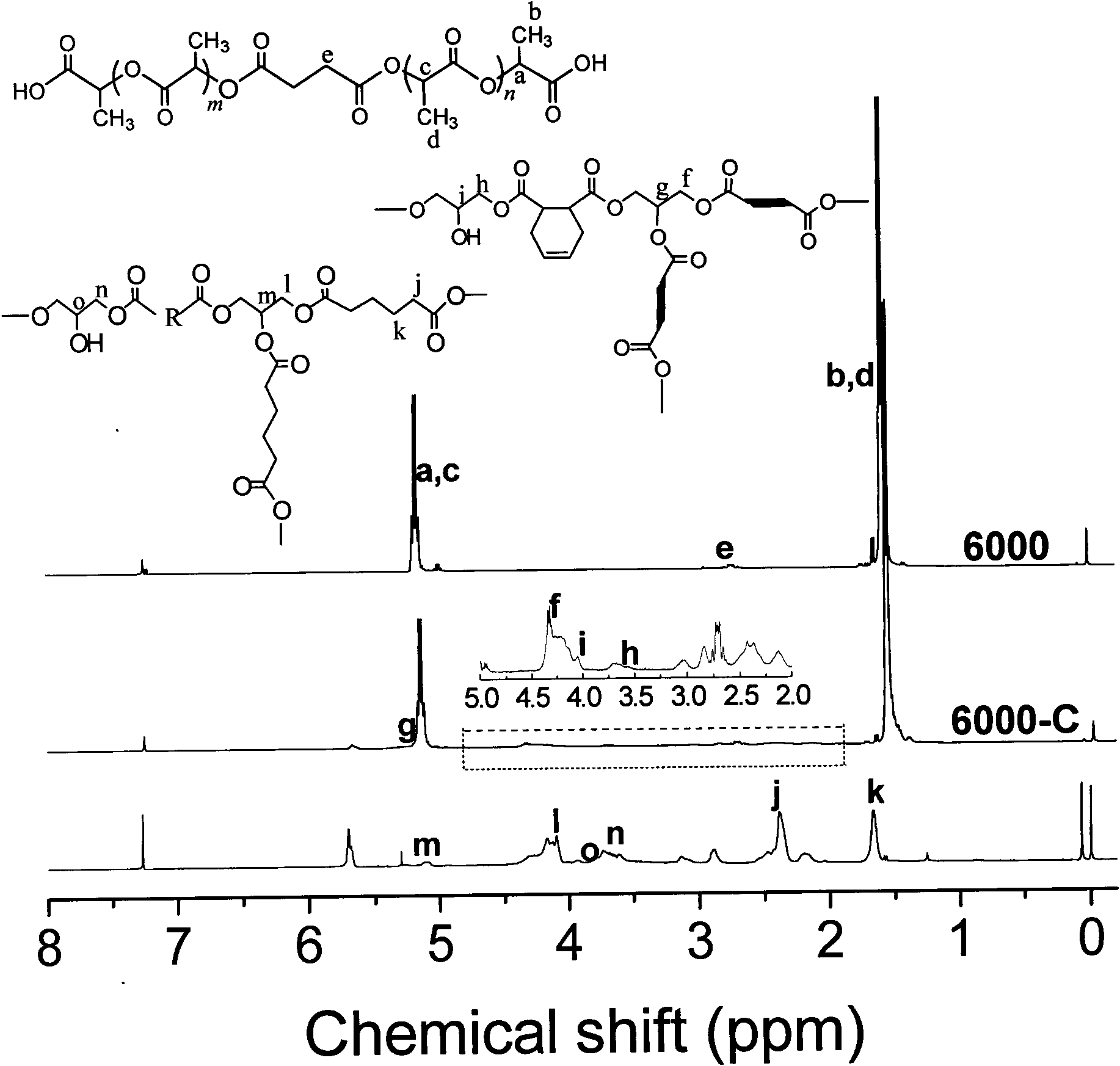
[0053] Take 180 grams of 90% L-lactic acid solution in a 250mL four-necked flask, connect the condensing device and the mechanical stirring device, add benzenesulfonic acid with a weight percentage content of 0.1% of the lactic acid weight, and heat in an oil bath. dehydration at lower temperature for 4 hours; then add succinic anhydride with 2% mole percentage of lactic acid, raise the temperature to 140°C, lower the pressure to 3000Pa, dehydrate and oligomerize for 4 hours; fill with nitrogen to relieve pressure, and add 0.1% by weight of lactic acid For stannous chloride, the temperature was raised to 160°C, the pressure was lowered to 300Pa, the melt polycondensed for 5 hours, and then the material was discharged and cooled to obtain a carboxyl-terminated polylactic acid prepolymer. Its number average molecular weight is 4000, the carboxyl terminal content is 98.9%, and the crystallinity is 27.4%.
[0054]Take 20g of the above-mentioned carboxyl-terminated polylactic acid ...
Embodiment 2
[0056] The preparation of carboxyl-terminated polylactic acid prepolymer is the same as in Example 1.
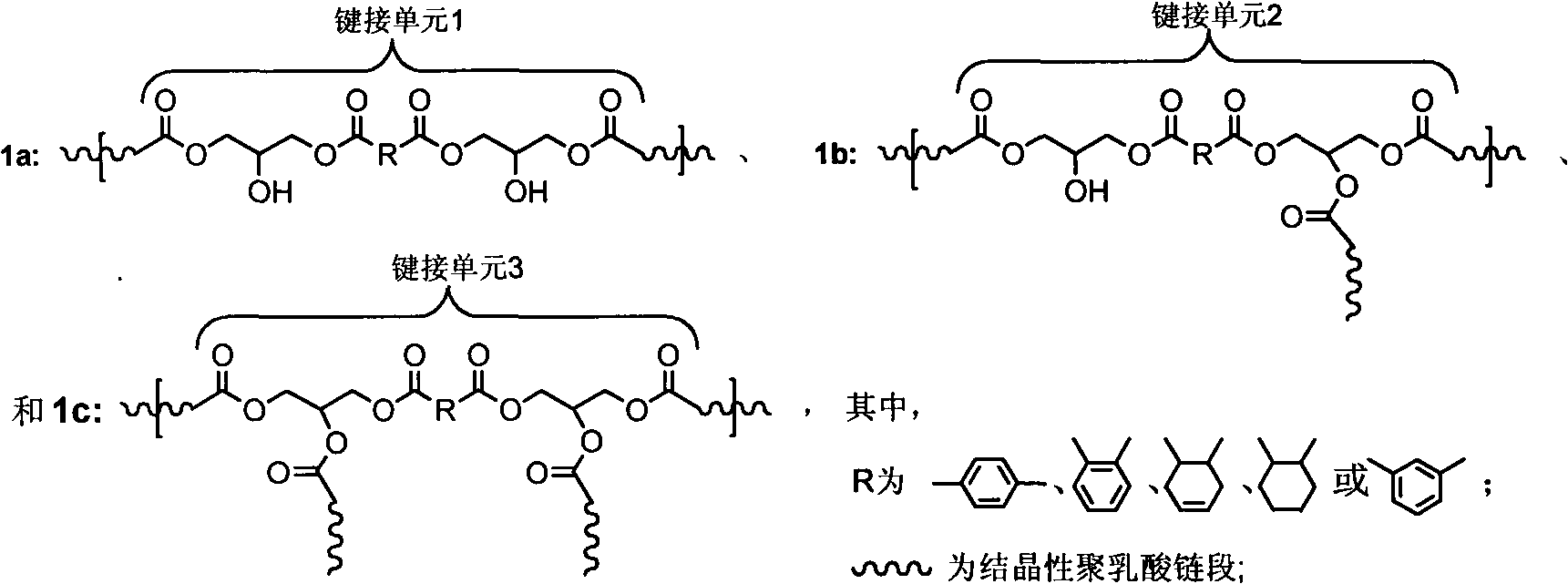
[0057] Take 20g of the above-mentioned carboxyl-terminated polylactic acid prepolymer and put it into a 100mL three-necked flask. Before the reaction, pass nitrogen to remove the air in the bottle, install a mechanical stirring device, and continue to pass nitrogen protection. After the prepolymer is completely melted, add the carboxyl molar ratio Diglycidyl tetrahydrophthalate of 1 was reacted at 160°C for 60 minutes to obtain white chain-extended polylactic acid. Its weight-average molecular weight is 123,000, the gel content is 3.4%, the branching factor is 0.35, and the crystallinity is 5%.
Embodiment 3
[0059] Take 180 grams of 90% L-lactic acid solution and put it into a 250mL four-neck flask, connect the condensing device and the mechanical stirring device, add p-toluenesulfonic acid with a weight percentage of 0.2% by weight of lactic acid, and heat in an oil bath at 130°C , Dehydration at 3000P for 3 hours; pressurize with nitrogen, add 1,6-adipic acid with a molar percentage of lactic acid of 1.2%, gradually raise the oil temperature to 150°C, drop the pressure to 3000Pa, react for 3h; fill with nitrogen to release , adding stannous chloride with a weight percentage of lactic acid weight of 0.2%, gradually increasing the temperature to 180°C, reducing the pressure to 200Pa, and performing melt polycondensation for 7 hours to obtain a carboxyl-terminated polylactic acid prepolymer. Its number average molecular weight is 6300, the carboxyl terminal content is 97.7%, and the crystallinity is 36.2%.
[0060] Take 20g of the above-mentioned carboxyl-terminated polylactic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com