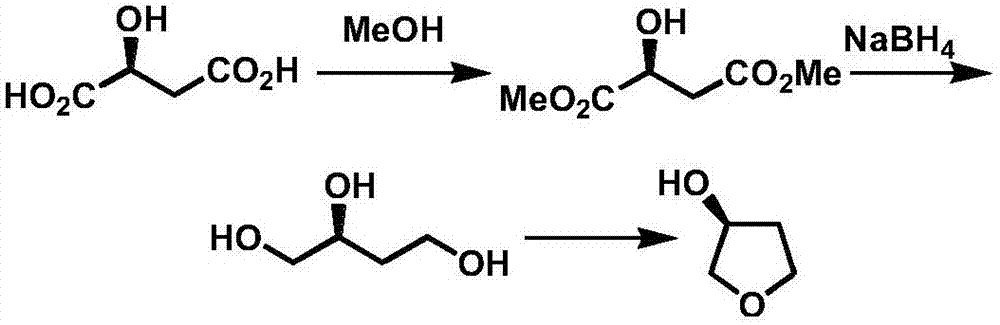
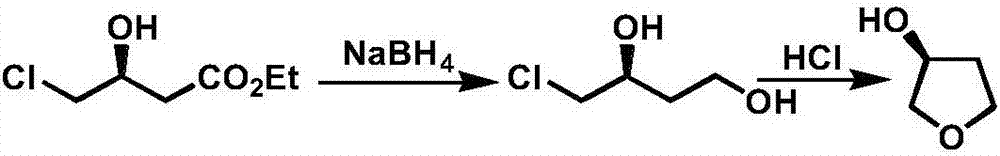
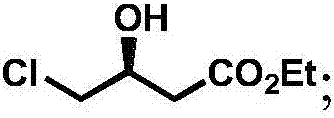
Production technology of 3-hydroxytetrahydrofuran with high optical purity
A technology of hydroxytetrahydrofuran and optical purity, which is applied in the field of preparation of pharmaceutical and pesticide intermediates, can solve the problems of low yield, complex reaction operation, high cost, etc., and achieve the effects of inhibiting racemization, simple and convenient operation, and inhibiting side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of chiral ethyl 4-chloro-3-hydroxybutyrate:
[0034] Add 500g (3.03mol) ethyl chloroacetoacetate and 2 liters of ethanol in 5 liters of reaction bottles, add chiral catalyst (0.15mol, 0.05eq.) and 250g (3.68mol, 1.2eq.) sodium formate after stirring, heat The temperature was raised to 50°C for reaction; gas phase detection was complete, the solvent was distilled off, and then 485 g of chiral ethyl 4-chloro-3-hydroxybutyrate was obtained by distillation under reduced pressure;
[0035] (2) Preparation of chiral 4-chloro-3-hydroxyl-1-butanol:
[0036] Add 110g (2.9mol, 1eq.) sodium borohydride and 2 liters of tetrahydrofuran into a 5-liter reaction flask, and slowly add 485g (2.9mol, 1eq.) chiral 4-chloro-3-hydroxybutyric acid dropwise at a temperature of 40°C Ethyl ester; after the dropwise addition, heat to reflux for 2 hours, cool down, use dilute hydrochloric acid to extract the reaction, filter with suction, and distill the filtrate to remove the sol...
Embodiment 2
[0040] (1) Preparation of chiral ethyl 4-chloro-3-hydroxybutyrate:
[0041] Add 500g (3.03mol) ethyl chloroacetoacetate and 2 liters of methanol into a 5-liter reaction flask, stir evenly, add a chiral catalyst (0.3mol, 0.1eq.), control the temperature at about 25°C, and add dropwise 400ml of formic acid / Triethylamine (5:2mol / mol) adducts, maintain this temperature to continue the reaction; the gas phase detection reaction is complete, first distill and recover methanol and formic acid / triethylamine adducts, the residue is then distilled under reduced pressure to obtain 488g chiral Ethyl 4-chloro-3-hydroxybutyrate.
[0042] (2) Preparation of chiral 3-hydroxytetrahydrofuran:
[0043] Add 122g (3.21mol, 1.1eq.) sodium borohydride and 2 liters of ethylene glycol dimethyl ether into a 5-liter reaction flask, and slowly add 488g (2.92mol, 1eq.) chiral 4-chloro -3-Hydroxybutyric acid ethyl ester; after the dropwise addition, continue to control the temperature at 50°C for 5 hour...
Embodiment 3
[0045] (1) Preparation of chiral ethyl 4-chloro-3-hydroxybutyrate:
[0046]Add 500g (3.03mol) ethyl chloroacetoacetate and 2 liters of isopropanol in 5 liters of reaction flasks, add chiral catalyst (0.15mol, 0.05eq.) and 286g (4.55mol, 1.5eq.) formic acid after stirring Ammonium, heated to 40 ° C to react; gas phase detection reaction is complete, distilled to remove the solvent, and then distilled under reduced pressure to obtain 501g chiral ethyl 4-chloro-3-hydroxybutyrate;
[0047] (2) Preparation of chiral 4-chloro-3-hydroxyl-1-butanol:
[0048] Add 195g (3.6mol, 1.2eq.) potassium borohydride and 2 liters of tetrahydrofuran into a 5-liter reaction flask, and slowly add 501g (3mol, 1eq.) chiral 4-chloro-3-hydroxybutyric acid dropwise at a temperature of 40°C Ethyl ester; after the dropwise addition, heat to reflux for 4 hours, cool, use dilute hydrochloric acid to extract the reaction, filter with suction, and distill the filtrate to remove the solvent to obtain chiral 4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com