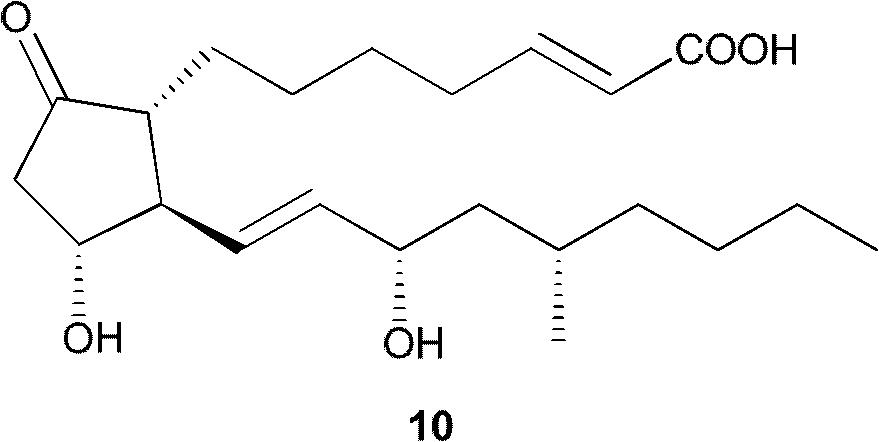
Method for preparing key intermediate of limaprost
A technology of limatoprost and branched chain alkyl, which is applied in the field of preparation of chiral compounds, can solve problems such as low production efficiency, large solvent, and difficulty in scaling up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
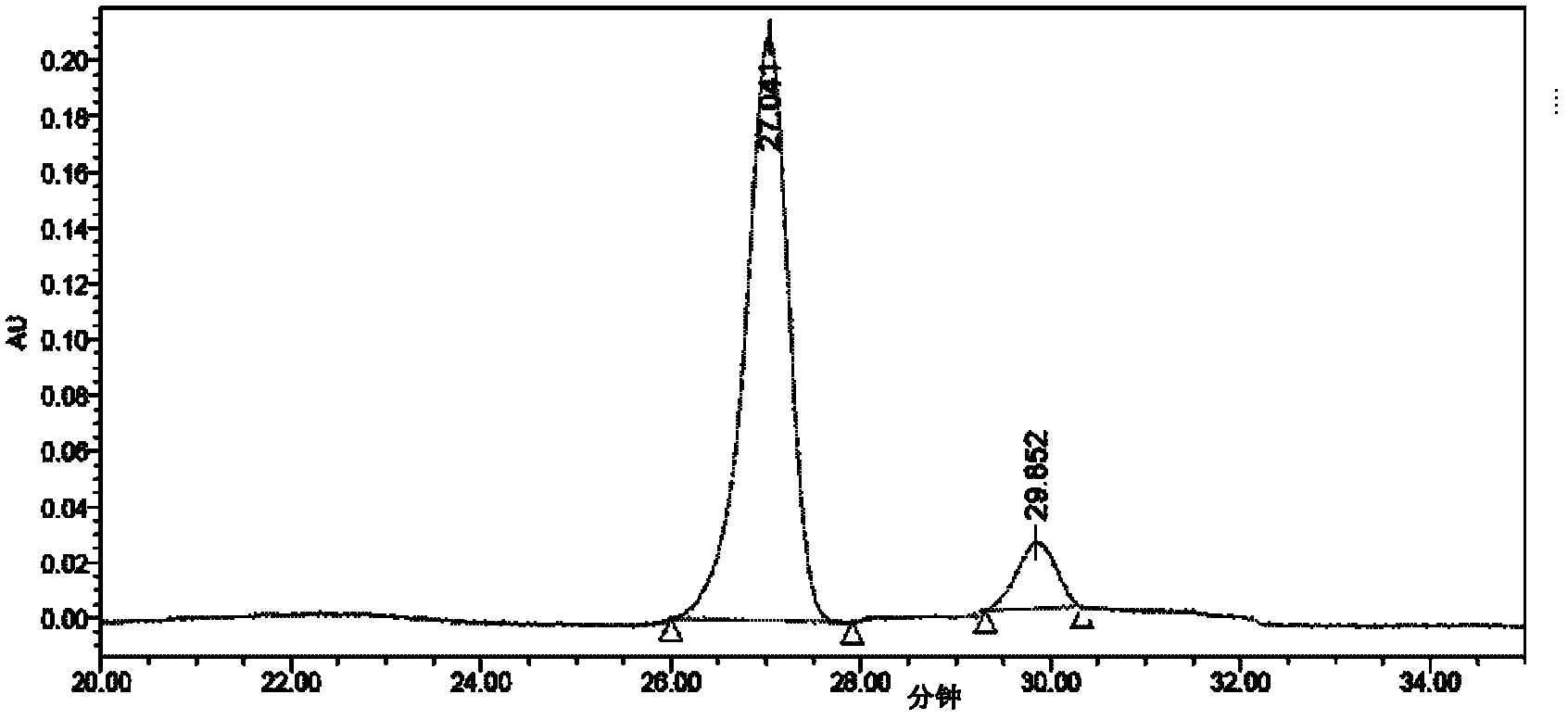
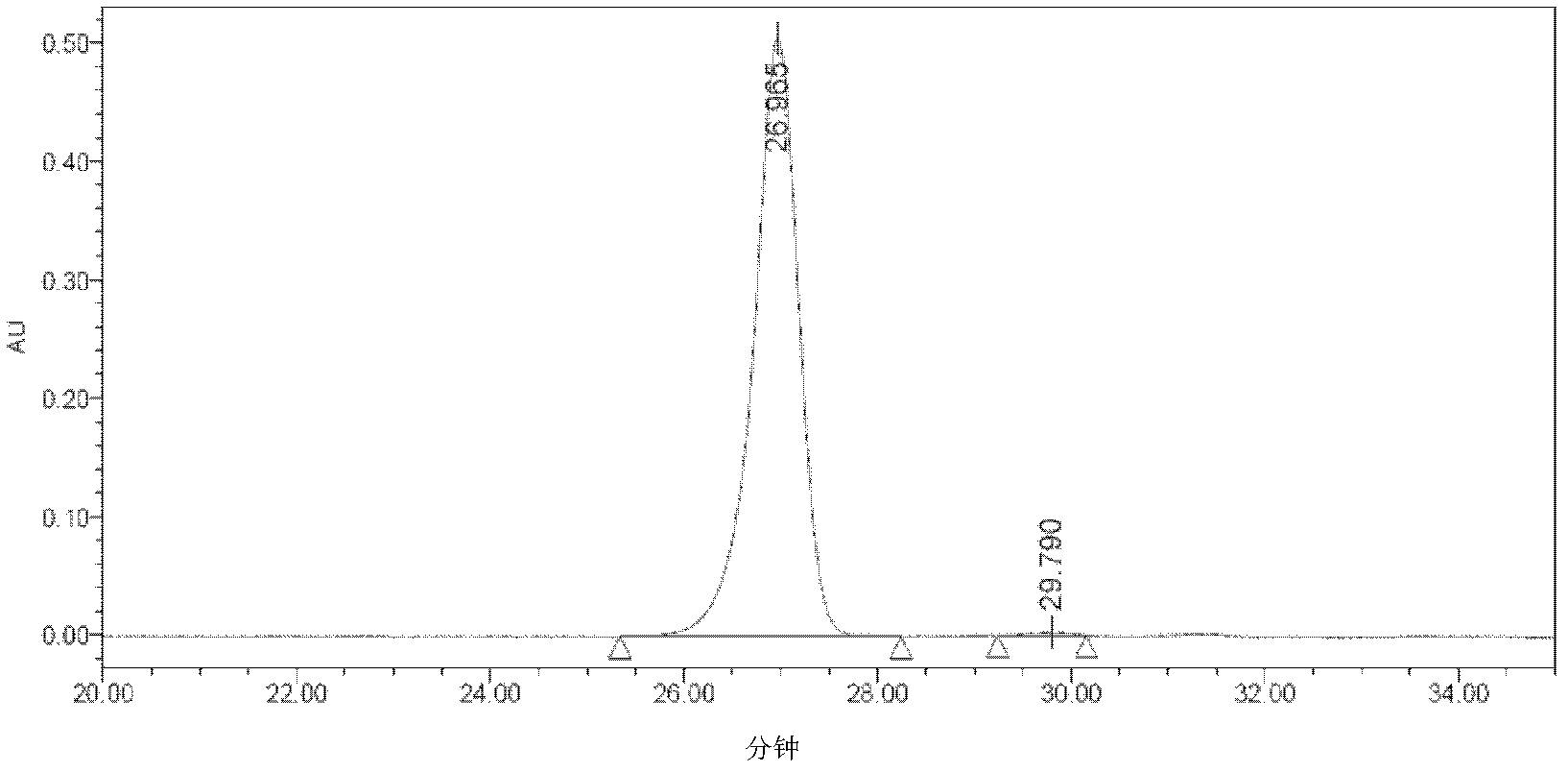
Image
Examples
preparation example Construction
[0098] The preparation of formula 1 intermediate and formula 2 intermediate
[0099] The formula 1 intermediate and the formula 2 intermediate of the present invention can be prepared by the following method, but the conditions of the method, such as the amount of reactant, solvent, base, compound used, reaction temperature, reaction time required, etc. are not limited to the following Explanation. The compound of the present invention can also be conveniently prepared by optionally combining various synthetic methods described in the specification or known in the art. Such a combination can be easily performed by those skilled in the art to which the present invention belongs.
[0100] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent, and the reaction time is usually 0.1-48 hours, preferably 0.5-24 hours.
[0101] Taking the intermediate of formula 2 as an example, a preferred method includes:
[0102] (1) under ac...
Embodiment 1
[0145] Preparation of starting material formula 8a compound
[0146] The commercially available Corey lactone is used as a starting material, and it is prepared by referring to the methods reported in documents GB1579464A1 and US3992439A1.
Embodiment 3
[0154] Preparation of formula 1a compound
[0155]
[0156] The compound of formula 3 (8.23g), the compound of formula 4a (6.6g), concentrated sulfuric acid (0.2ml) and toluene (40ml) were stirred evenly, heated to reflux for dehydration reaction for 5h, followed by TLC. After complete reaction, lower the reaction temperature and add 10% Na 2 CO 3 (4ml) to quench the reaction, extract the aqueous layer with n-hexane (20ml*4), combine the organic layers and wash with saturated sodium chloride solution (50ml), dry over anhydrous sodium sulfate and distill under reduced pressure to collect fractions at 120-126°C , to obtain the compound of formula 5a (13.9 g).
[0157] Under the protection of nitrogen, the compound of formula 6a (14.2g) and tetrahydrofuran (83ml) were mixed, stirred and cooled to below -70°C, and n-hexane solution of n-Butyl lithium (n-Butyl lithium) (45.7ml, 2.5M ), the dropwise addition process controlled the temperature below -70°C, and after the dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com