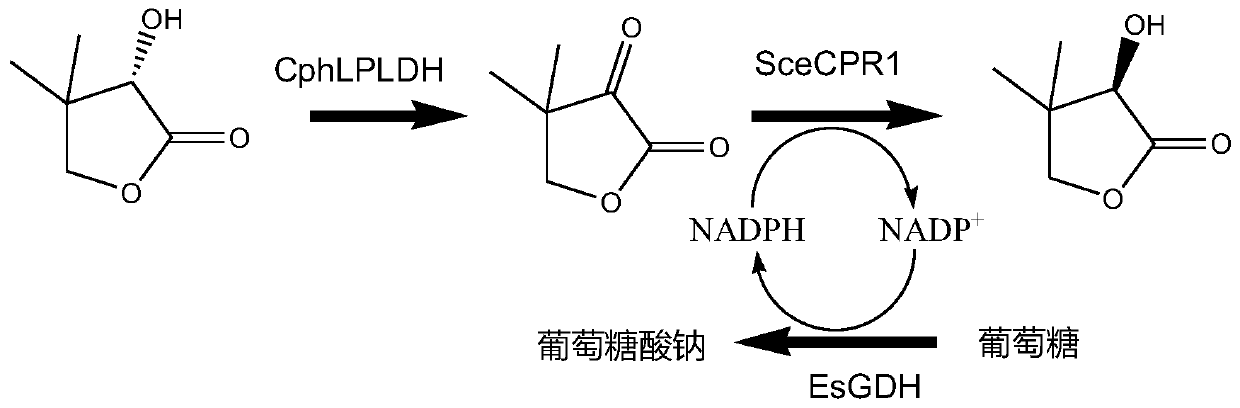
L-pantoyl lactone dehydrogenase derived from Cnuibacter physcomitrellae
A technology of pantolactone and ester dehydrogenase, which is applied in the direction of enzymes, oxidoreductases, and microbial-based methods, can solve the problems of unknown coding genes and hinder applications, so as to reduce the use of acid and alkali, Simplify the reaction process and avoid cumulative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
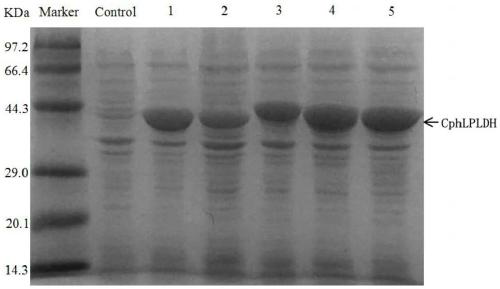
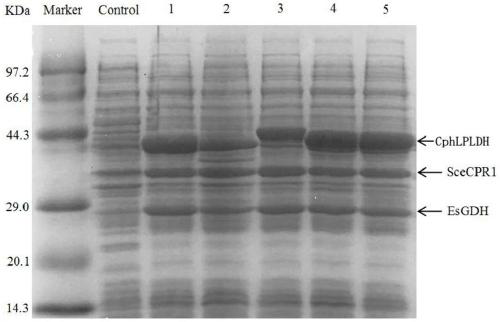
[0072] Example 1 Construction and expression of L-pantolactone dehydrogenase genetically engineered bacteria
[0073] Using the published oxidoreductase encoding gene from Cnuibacter physcomitrellae (GenBank accession number is ARJ05030.1), after codon optimization, L-pantoic acid was artificially synthesized (gene synthesis service provided by Suzhou Jinweizhi Biotechnology Co., Ltd.) The gene encoding lactone dehydrogenase has a nucleotide sequence as shown in SEQ ID NO:1.
[0074] The gene encoding L-pantolactone dehydrogenase (CphLPLDH) derived from Cnuibacter physcomitrellae was inserted into the HindIII and XhoI sites of plasmid pET-28b to obtain recombinant plasmid pET-28b-CphLPLDH. Transfer pET-28b-CphLPLDH into E.coli BL21(DE3) to obtain genetic engineering bacteria E.coli BL21(DE3) / pET-28b-CphLPLDH.
[0075] The genetically engineered bacteria E.coli BL21(DE3) / pET-28b-CphLPLDH were inoculated in LB liquid medium containing a final concentration of 100 μg / mL kanamyci...
Embodiment 2
[0076] The investigation of the substrate specificity of embodiment 2L-pantolactone dehydrogenase
[0077] The wet thallus obtained after the induced expression of genetically engineered bacteria E.coli BL21(DE3) / pET-28b-CphLPLDH was used as a biocatalyst. Using 100mM D-pantoate lactone, L-pantoate lactone, DL-pantoate lactone, D-pantoate and L-pantoate as substrates, the whole-cell catalysis was used to investigate the origin of Substrate specificity of the L-pantolactone dehydrogenase of Cnuibacter physcomitrellae. The reaction system for the dehydrogenation of L-pantolactone dehydrogenase catalyzed by L-pantolactone dehydrogenase is 5 mL, containing respectively: 1 g of wet bacteria, 100 mM substrate and 200 mM phosphate buffer (pH 7.0). The reaction solution was added to a three-necked flask, and the reaction conditions were maintained at 30°C, 600rpm and pH 7.0 under magnetic stirring, and the catalytic process was added dropwise with 1M Na 2 CO 3 The solution maintain...
Embodiment 3
[0081] Example 3D- Acquisition of Ketopantolactone Reductase Encoding Gene
[0082] Using the published reductase encoding gene (GenBank accession number: CAA98692.1) derived from Saccharomyces cerevisiae, after codon optimization, artificially synthesized (gene synthesis service provided by Suzhou Jinweizhi Biotechnology Co., Ltd.) D- The gene encoding ketopantolactone reductase has a nucleotide sequence as shown in SEQ ID NO:3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com