Synthesis method of enantiomer-enriched indoline-2-formic acid
A synthetic method, the technology of indoline, applied in the direction of organic chemistry, can solve the problems of low utilization rate of chiral split atoms, large pollution, high cost of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
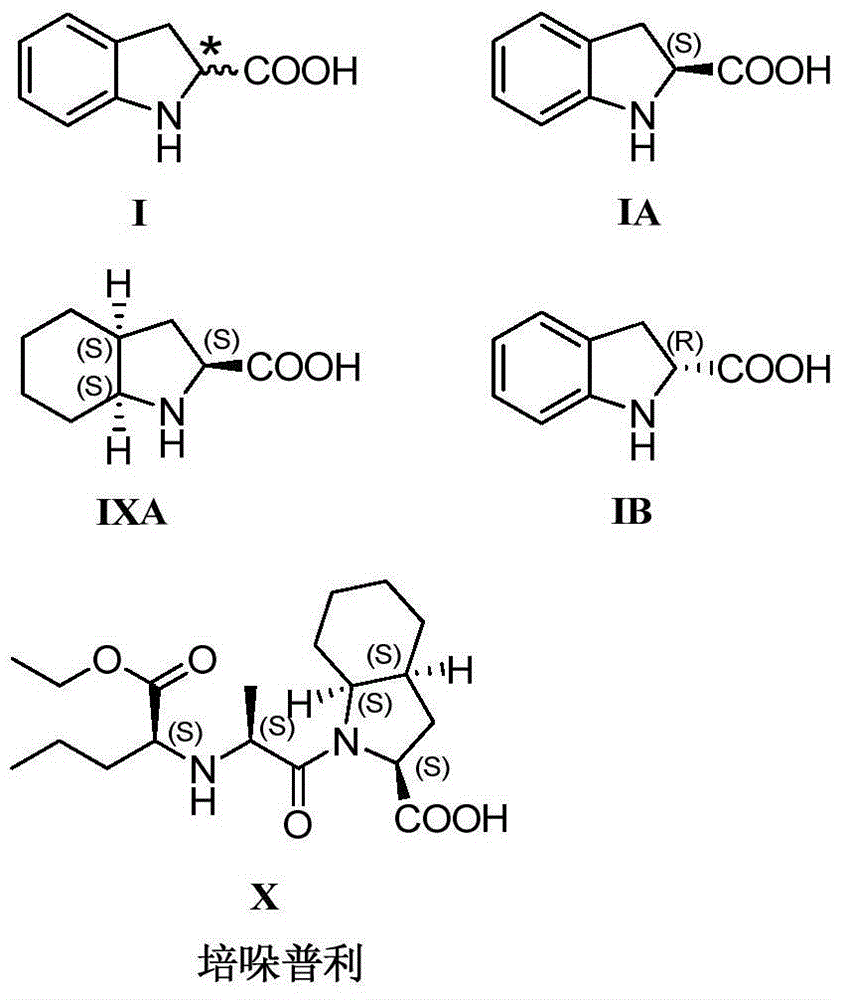
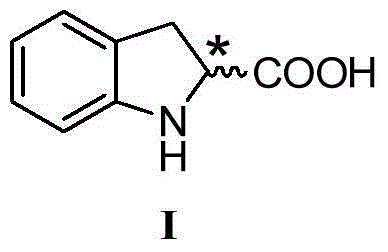
[0078] Example 1: (2S)-indoline-2-carboxylic acid (Formula IA)
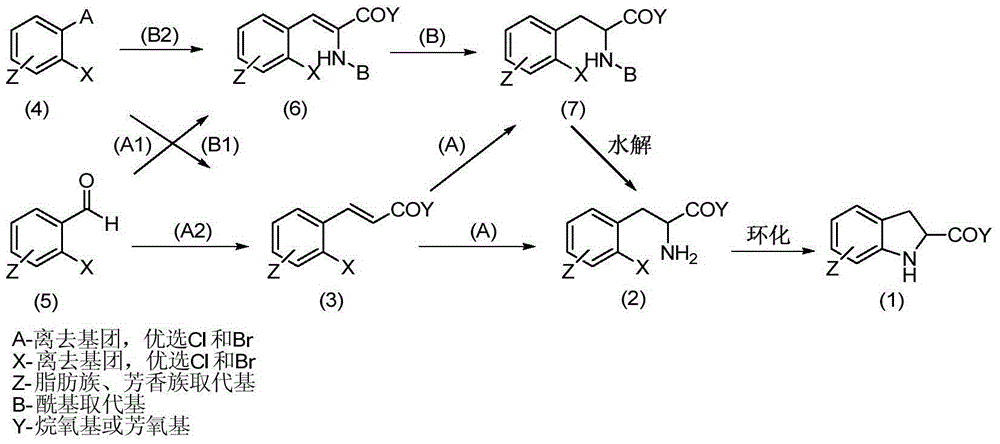
[0079] Step 1: (4Z)-4-[(2-Bromophenyl)methylene]-2-phenyl-1,3-oxazol-5-one
[0080] Under nitrogen protection, N-benzoylglycine (0.5mol, 89.6g), anhydrous potassium acetate (0.55mol, 54.0g), and acetic anhydride (2.5mol, 255.2g) were successively dropped into a 1L three-necked flask. Stir mechanically for 30 minutes, and the reaction system is a solid-liquid system. Then, slowly add o-bromobenzaldehyde (0.55mol, 101.8g), heat up to 100°C, keep the temperature of the reaction system at 90-100°C, and there is a slight reflux phenomenon. The reaction system gradually dissolved, and the color of the reaction solution deepened, showing wine red. TLC tracking (developer: n-hexane: ethyl acetate = 10:1) until the reaction at the raw material point was complete. Turn off the heating, cool down to 0-5°C in an ice bath, a large amount of yellow solid precipitates, and stand for 6 hours for crystallization. Suction filtr...
Embodiment 2
[0093] Example 2: (2S)-indoline-2-carboxylic acid (Formula IA)
[0094] Step 1: (4Z)-4-[(2-Chlorophenyl)methylene]-2-methyl-1,3-oxazol-5-one
[0095] Under nitrogen protection, N-acetylglycine (0.5mol, 58.6g), anhydrous sodium acetate (0.55mol, 45.1g), acetic anhydride (1.5mol, 154.4g) were successively dropped into a 1L three-necked flask, and mechanically stirred at room temperature 30min, the reaction system is a solid-liquid system. Then, slowly add o-chlorobenzaldehyde (0.55mol, 77.3g), heat up to 100°C, keep the temperature of the reaction system at 90-100°C, and there is a slight reflux phenomenon. The reaction system gradually dissolved, and the color of the reaction solution deepened, showing wine red. TLC tracking (developer: n-hexane: ethyl acetate = 10:1) until the reaction at the raw material point was complete. Turn off the heating, naturally cool down to 50°C, add 150mL of n-hexane to dilute, and a yellow solid precipitates out. Then the temperature was lower...
Embodiment 3
[0108] Example 3: (2R)-indoline-2-carboxylic acid (Formula IB)
[0109] Step 1: (4Z)-4-[(2-Chlorophenyl)methylene]-2-methyl-1,3-oxazol-5-one
[0110] Under nitrogen protection, N-acetylglycine (0.5mol, 58.6g), anhydrous potassium acetate (0.55mol, 54.0g), and acetic anhydride (1.5mol, 154.4g) were successively dropped into a 1L three-necked flask, and mechanically stirred at room temperature 30min, the reaction system is a solid-liquid system. Then, slowly add o-chlorobenzaldehyde (0.55mol, 77.3g), heat up to 100°C, keep the temperature of the reaction system at 90-100°C, and there is a slight reflux phenomenon. The reaction system gradually dissolved, and the color of the reaction solution deepened, showing wine red. TLC tracking (developer: n-hexane: ethyl acetate = 10:1) until the reaction at the raw material point was complete. Turn off the heating, naturally cool down to 50°C, add 150mL of n-hexane to dilute, and a yellow solid precipitates out. Then the temperature wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com