Method for enzymatically synthesizing chiral acetic cyanhydrin ester by directly using cyanogen salt as cyanogens source
A cyanohydrin acetate and a technology using cyanide salts are applied in the field of enzyme-catalyzed synthesis of chiral cyanohydrin acetate, which can solve the problems of decreased optical purity of the product, loss of application value and the like, and achieves the effects of high industrial application value and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: ( R )-α-Acetoxy-2-Phenylacetonitrile Preparation
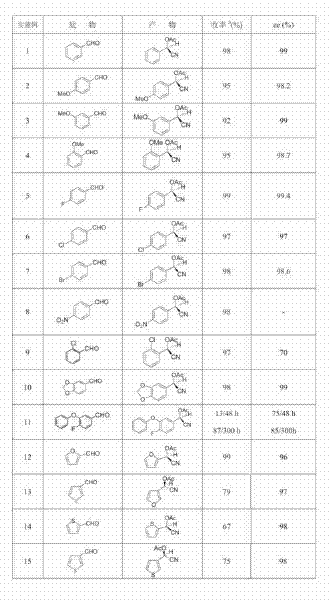
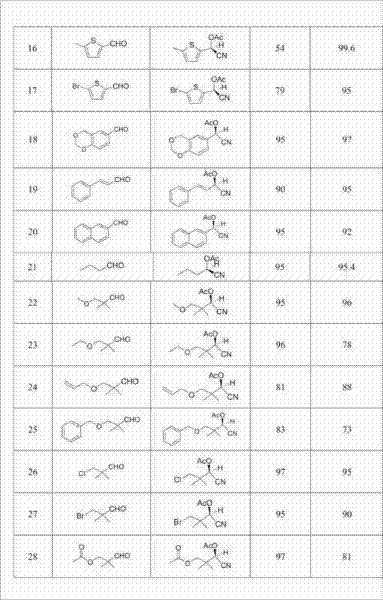
[0023] Add 1 g of almond enzyme, 6 mmol of NaCN, and 7 mmol of acetic acid to 1 mL of water and 9 mL of ethyl acetate, stir well, add 3 mmol of benzaldehyde dropwise, and react for 48 hours; the organic phase obtained after liquid separation is added dropwise to In a mixture containing 6 mmol acetic anhydride and 6 mmol pyridine, react at room temperature for 3 hours. After being washed with saturated sodium bicarbonate and water, dried, concentrated and purified by column chromatography, the target compound was obtained, and the chemical yield was calculated. The content of the two optical isomers of the product was determined by a chiral chromatographic column and the enantiomeric excess percentage (ee value) was calculated. The results are shown in Table 1.
Embodiment 2
[0024] Embodiment 2: ( R )-α-Acetoxy-2-(4-methoxyphenyl)acetonitrile
[0025] In 5 mL of water and 5 mL of ethyl acetate, add 1 g of almond enzyme, 6 mmol of KCN, and 10 mmol of acetic acid, stir well, add 3 mmol of 4-methoxybenzaldehyde dropwise, and react for 24 hours; The organic phase was added dropwise to a mixture containing 6 mmol acetic anhydride and 6 mmol pyridine, and reacted at room temperature for 5 hours. After being washed with saturated sodium bicarbonate and water, dried, concentrated and purified by column chromatography, the target compound was obtained, and the chemical yield was calculated. The content of the two optical isomers of the product was determined by a chiral chromatographic column and the enantiomeric excess percentage (ee value) was calculated. The results are shown in Table 1.
Embodiment 3
[0026] Embodiment 3: ( R )-α-Acetoxy-2-(3-methoxyphenyl)acetonitrile preparation
[0027] Add 1.5 g of laetrile, 5 mmol of NaCN, and 5 mmol of acetic acid to 5 mL of water and 5 mL of toluene, stir well, add 3 mmol of 3-methoxybenzaldehyde dropwise, and react for 48 hours; the organic phase obtained after liquid separation Add it dropwise to a mixture containing 10 mmol acetic anhydride and 10 mmol pyridine, and react at room temperature for 24 hours. After being washed with saturated sodium bicarbonate and water, dried, concentrated and purified by column chromatography, the target compound was obtained, and the chemical yield was calculated. The content of the two optical isomers of the product was determined by a chiral chromatographic column and the enantiomeric excess percentage (ee value) was calculated. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com