Method for synthesizing optical enantiomer 6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-carboxylic acid and 6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-carboxylate
A technology of benzopyran and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as low yield, large reaction pollution, unreasonable synthesis method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
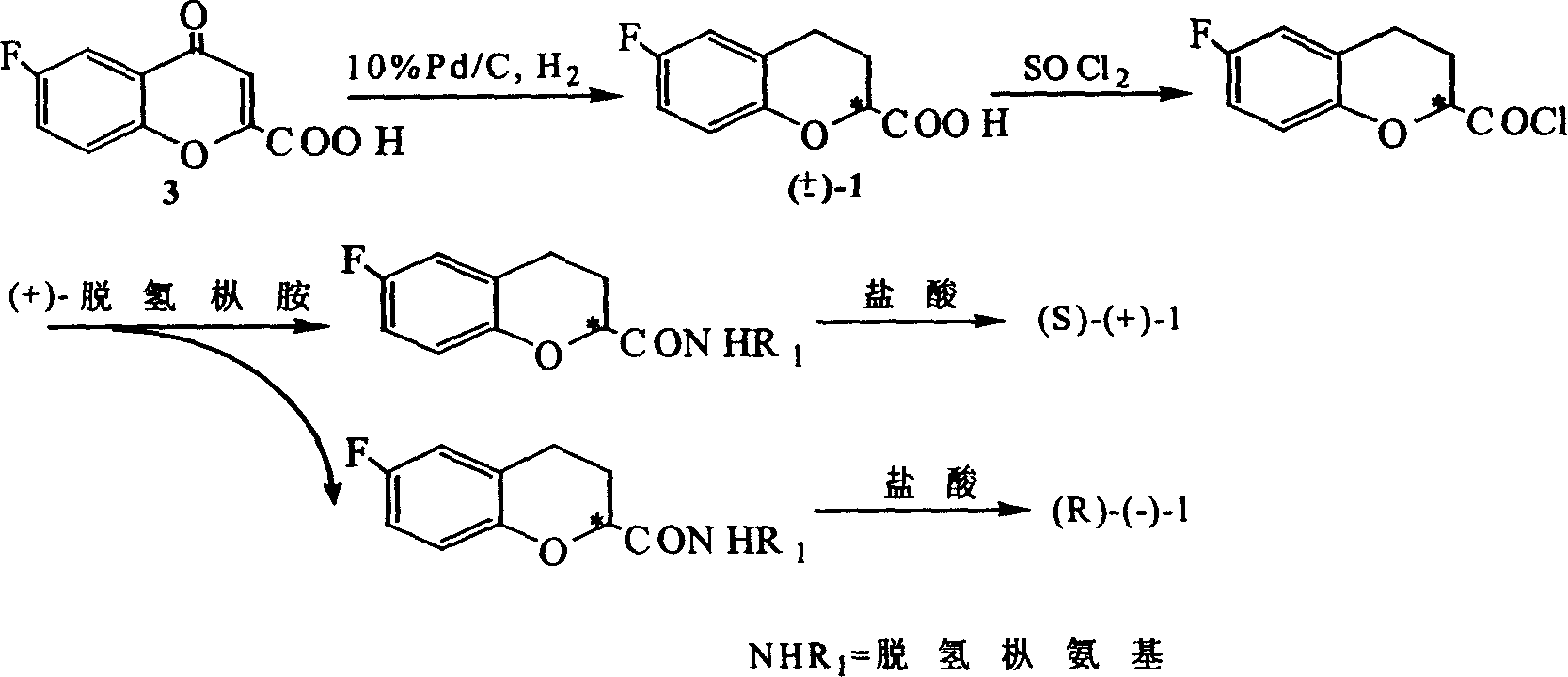
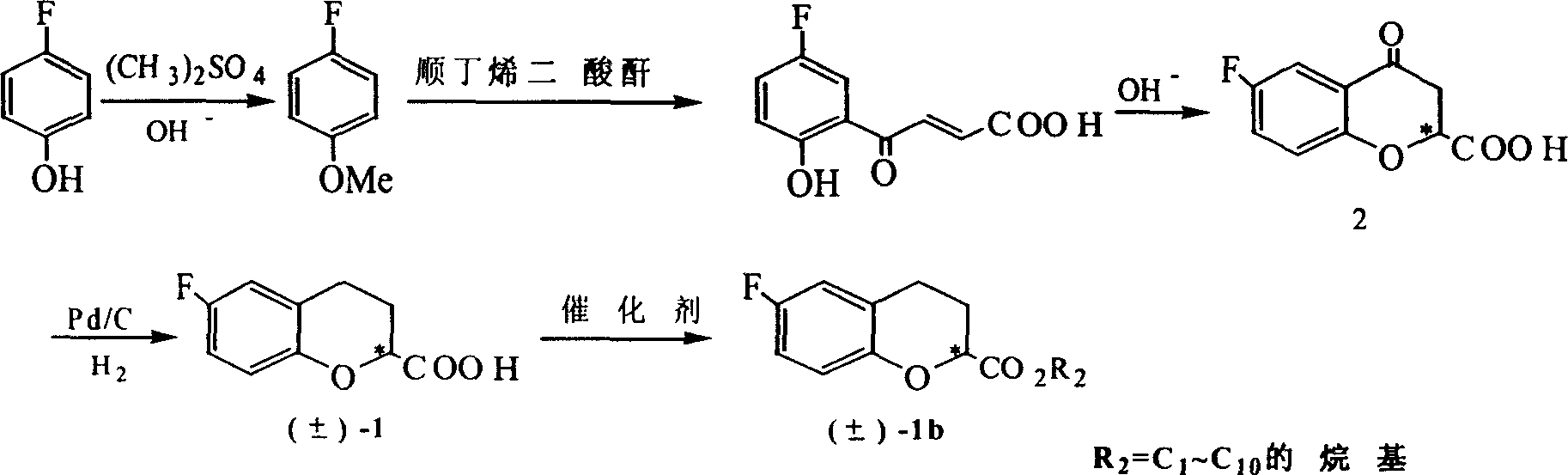
[0046] Example 1. Prepare (±)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid [in the formula (±)-1] and (±) according to synthetic method one -6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylate [where (±)-1b]
[0047] 1.1 p-Fluoroanisole
[0048] Dissolve p-fluorophenol (25.6g, 0.05mol) in 500ml of 5wt% NaOH aqueous solution, add dimethyl sulfate (32g, 0.254mol) dropwise, heat to 70°C, stir for 5 hours and stop, wait until the system is cooled to room temperature Then, it was extracted with ethyl acetate 20ml×3, the ethyl acetate layer was collected, dried with anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation to obtain a light yellow viscous liquid, which was distilled under reduced pressure to collect 38-40℃ / The 5mmHg fraction yielded p-fluoroanisole (6.2g, 98%).
[0049] 1.2 4-(5-Fluoro-2-hydroxy)phenyl-4one-2,3-butenoic acid
[0050] Feed with maleic anhydride (4g, 0.04mol), aluminum trichloride catalyst (15g, 0.112mol), p-fluoroani...
Embodiment 2
[0060] 1.1 p-Fluoroanisole
[0061] Dissolve p-fluorophenol (51.2g, 0.1mol) in 5wt% Na 2 CO 3 Add dimethyl sulfate (64g, 0.508mol) dropwise to 1000ml of aqueous solution, heat to 75°C, stir for 5 hours and then stop. After the system is cooled to room temperature, extract with 40ml×3 ethyl acetate and collect the ethyl acetate layer. After drying with anhydrous sodium sulfate, filtering, and rotary evaporation to remove the solvent, a light yellow viscous liquid was obtained, which was distilled under reduced pressure to collect the fraction of 38~40℃ / 5mmHg to obtain p-fluoroanisole (12.4g, 98%) .
[0062] 1.2 4-(5-Fluoro-2-hydroxy)phenyl-4one-2,3-butenoic acid
[0063] Feed with maleic anhydride (8g, 0.08mol), catalyst aluminum trichloride (30g, 0.224mol), p-fluoroanisole (5.0g, 0.04mol), dissolve in 55ml of dichloromethane, and heat to 60°C, The reaction is carried out for 2 hours. After the reaction is completed, a mixed solution of ice water and hydrochloric acid is added to t...
Embodiment 3
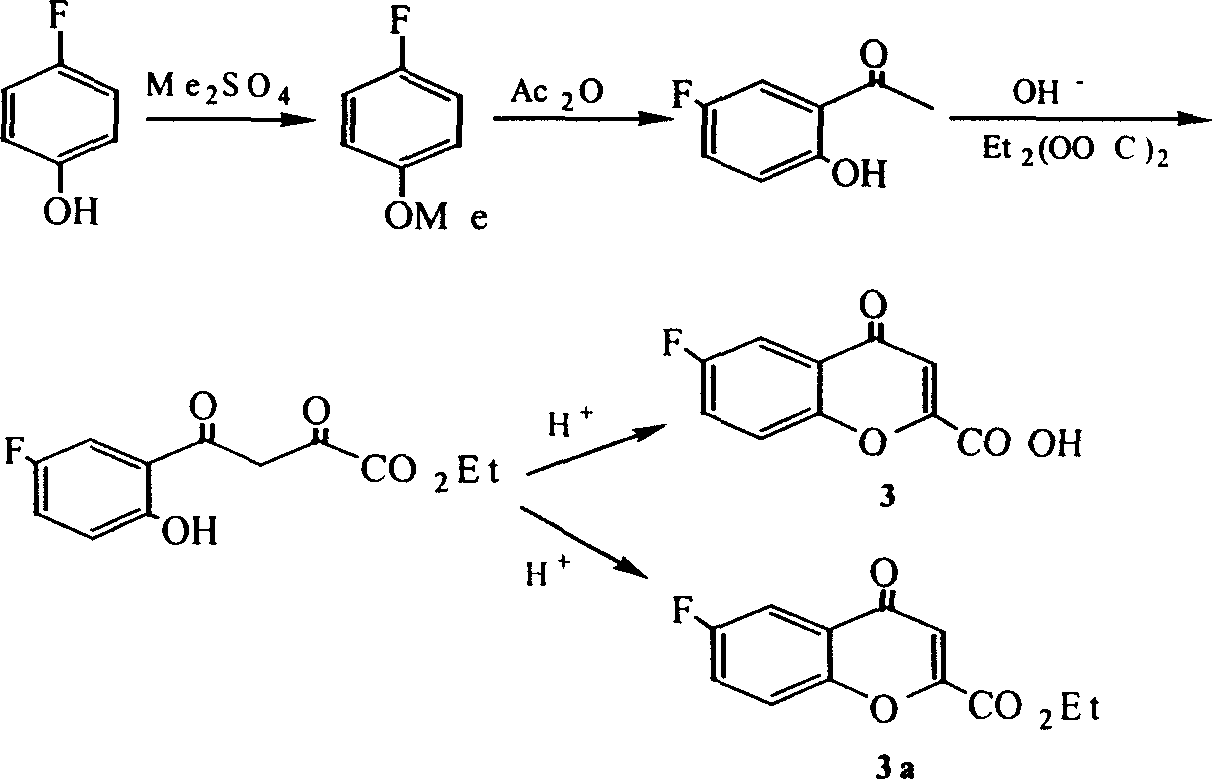
[0073] Prepare (±)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid [in the formula (±)-1] and (±)-6-fluoro -3,4-Dihydro-2H-1-benzopyran-2-carboxylate [where (±)-1b]
[0074] 1.1 p-Fluoroanisole
[0075] The preparation of p-fluoroanisole is the same as in Example 1.
[0076] 1.2 5-Fluoro-2-hydroxy-acetophenone
[0077] 4-Fluoroanisole (0.1mol), CS 2 100ml, catalyst aluminum trichloride (0.25mol), slowly add acetic anhydride (0.12mol) while stirring, the reaction temperature is 75℃, after the reaction is over, add appropriate amount of hydrochloric acid and crushed ice to the system, stir vigorously, divide The organic layer was separated, the aqueous layer was extracted with ethyl acetate (3×25 ml), the organic layers were combined and washed with water, and finally the organic layer was washed with Na 2 SO 4 After drying and removing the solvent, the product 5-fluoro-2-hydroxy-acetophenone is obtained. Mp 55~56℃.
[0078] 1.3(a) 5-Fluoro-2-hydroxy-acetophenone (0.1mol), di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com