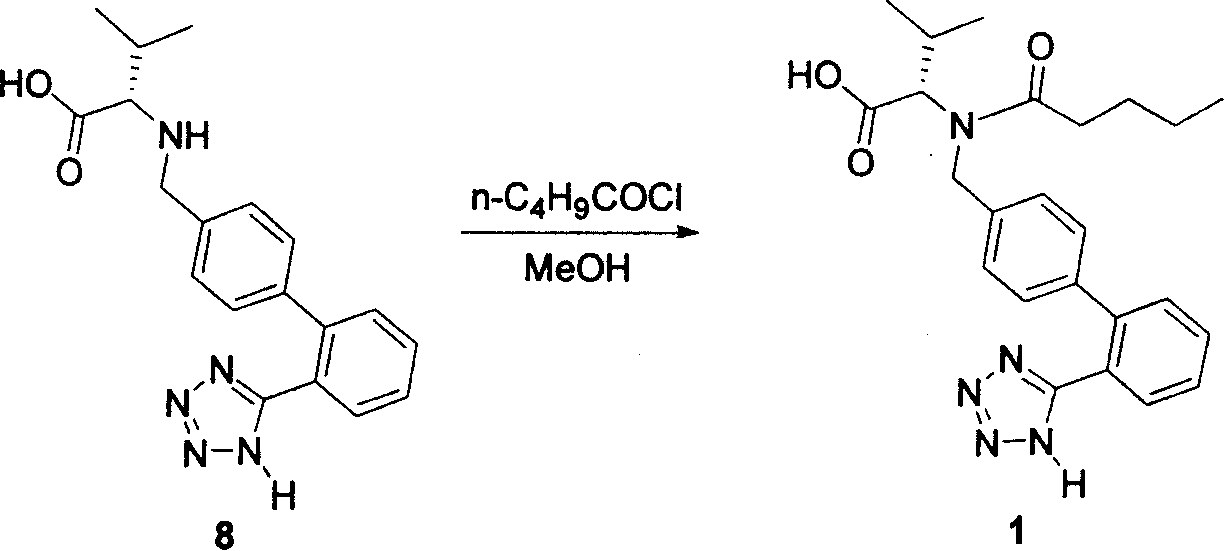
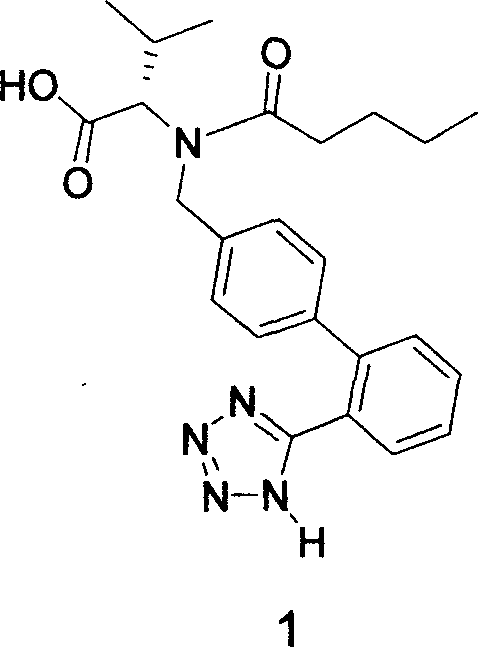
Method for synthesizing Valsartan with high optical purity
A technology of optical purity and valine, applied in the direction of organic chemistry, can solve the problems of cumbersome operation, high cost, and high toxicity of trialkyltin azide, and achieve the method of simple operation, inhibition of racemization, and optical purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
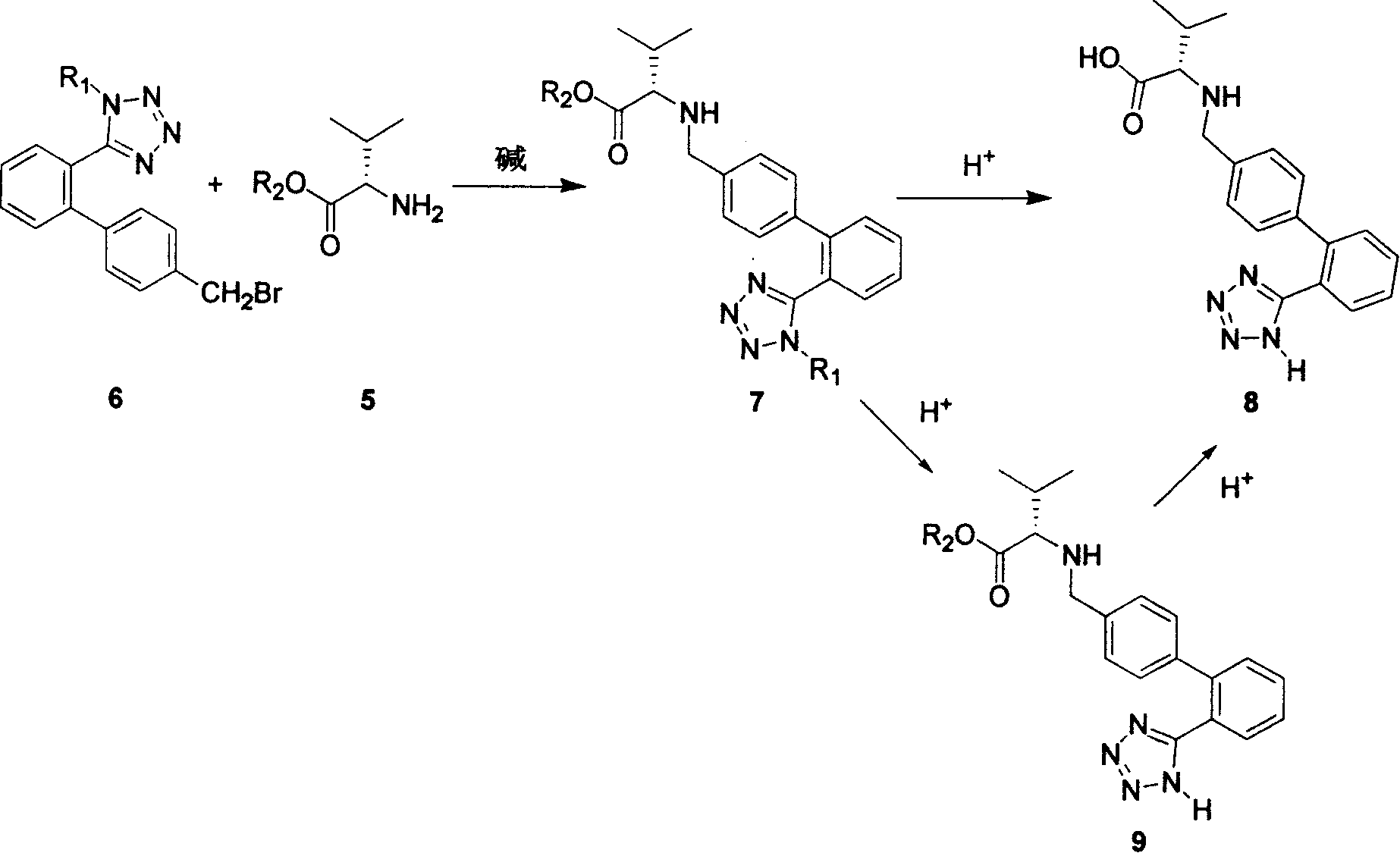
[0053] Example 1.N-[[2'-(2N-trityl-tetrazol-5-yl)-(1,1'-diphenyl)-4-yl]-methyl]-L- Valine methyl ester (7, R 1 = Trityl, R 2 = Methyl) Preparation:
[0054] In the 1000 milliliter four-necked bottle that is equipped with drying tube, thermometer, dropping funnel and mechanical stirring, add valine methyl ester (5, R 2 =methyl, 24.3 g), diisopropylethylamine (18.7 g), and dichloromethane (300 ml), stirred to dissolve, and cooled. At -10 to 0°C, drop N-trityl-2'-tetrazolyl-4-bromomethylbiphenyl (6, R 1 =trityl, 100 grams, 0.179mol) in dichloromethane (300 milliliters) solution; After adding, at 0~10 ℃, continue to react for 4 to 5 hours, TLC (developing solvent, n-hexane: ethyl acetate =5:1) shows that the raw material basically disappears; add 5% aqueous sodium bicarbonate solution to wash, wash with saturated brine, remove methylene chloride from the organic phase under reduced pressure, and obtain N-[[2'-(2N-trityl- Tetrazol-5-yl)-(1,1'-diphenyl)-4-yl]-methyl]-L-valine m...
Embodiment 2
[0055] Example 2. N-[[2'-(2N-trityl-tetrazol-5-yl)-(1,1'-diphenyl)-4-yl]-methyl]-L- Valine methyl ester (7, R 1 = Trityl, R 2 = Methyl) Preparation:
[0056] In the 1000 milliliter four-neck bottle that is equipped with drying tube, thermometer, dropping funnel and mechanical stirring, add valine methyl ester hydrochloride (5, R 2 =methyl, 31.1 g), dichloromethane (300 ml), stirred and cooled. At -10~0°C, diisopropylethylamine (42.6 g) was added dropwise, and the reaction was stirred for 0.5 hours; N-trityl-2'-tetrazolyl-4-bromomethylbis Benzene (6, R 1 =trityl, 100 grams, 0.179mol) in dichloromethane (300 milliliters) solution; After adding, at 0~10 ℃, continue to react for 4 to 5 hours, TLC (developing solvent, n-hexane: ethyl acetate =5:1) shows that the raw material basically disappears; add 5% aqueous sodium bicarbonate solution to wash, wash with saturated brine, remove methylene chloride from the organic phase under reduced pressure, and obtain N-[[2'-(2N-trityl- ...
Embodiment 3
[0057] Example 3. N-[[2'-(2N-trityl-tetrazol-5-yl)-(1,1'-diphenyl)-4-yl]-methyl]-L- Valine isopropyl ester (7, R 1 = Trityl, R 2 =isopropyl) Preparation:
[0058] In the 1000 milliliter four-necked bottle that is equipped with drying tube, thermometer, dropping funnel and mechanical stirring, add valine isopropyl ester (5, R 2 =isopropyl, 29.6 g), diisopropylethylamine (18.7 g), and dichloromethane (300 ml), stirred to dissolve, and cooled. At -10 to 0°C, drop N-trityl-2'-tetrazolyl-4-bromomethylbiphenyl (6, R 1 = trityl group, 100 grams) in dichloromethane (300 milliliters) solution; After adding, at 0~10 ℃, continue to react for 4 to 5 hours, TLC (developing solvent, n-hexane: ethyl acetate=5: 1) It shows that the raw material basically disappears; add 5% aqueous sodium bicarbonate solution to wash, wash with saturated brine, remove dichloromethane from the organic phase under reduced pressure, and obtain N-[[2'-(2N-trityl-tetrazolium -5-yl)-(1,1'-diphenyl)-4-yl]-methyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com