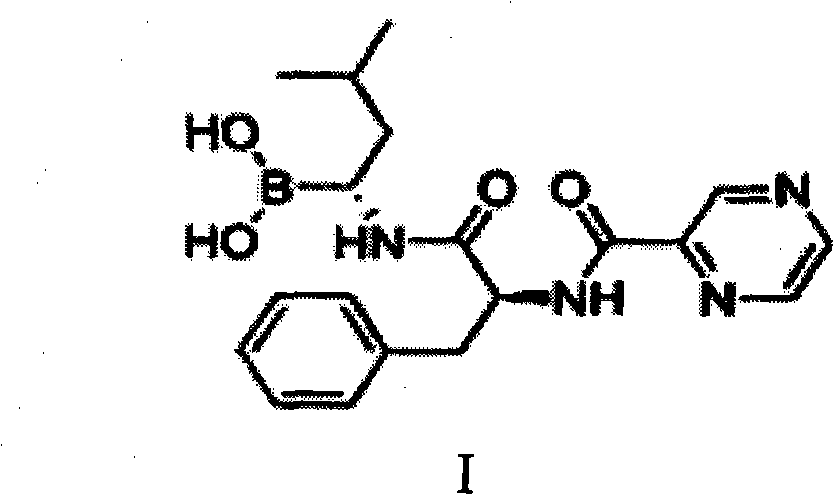
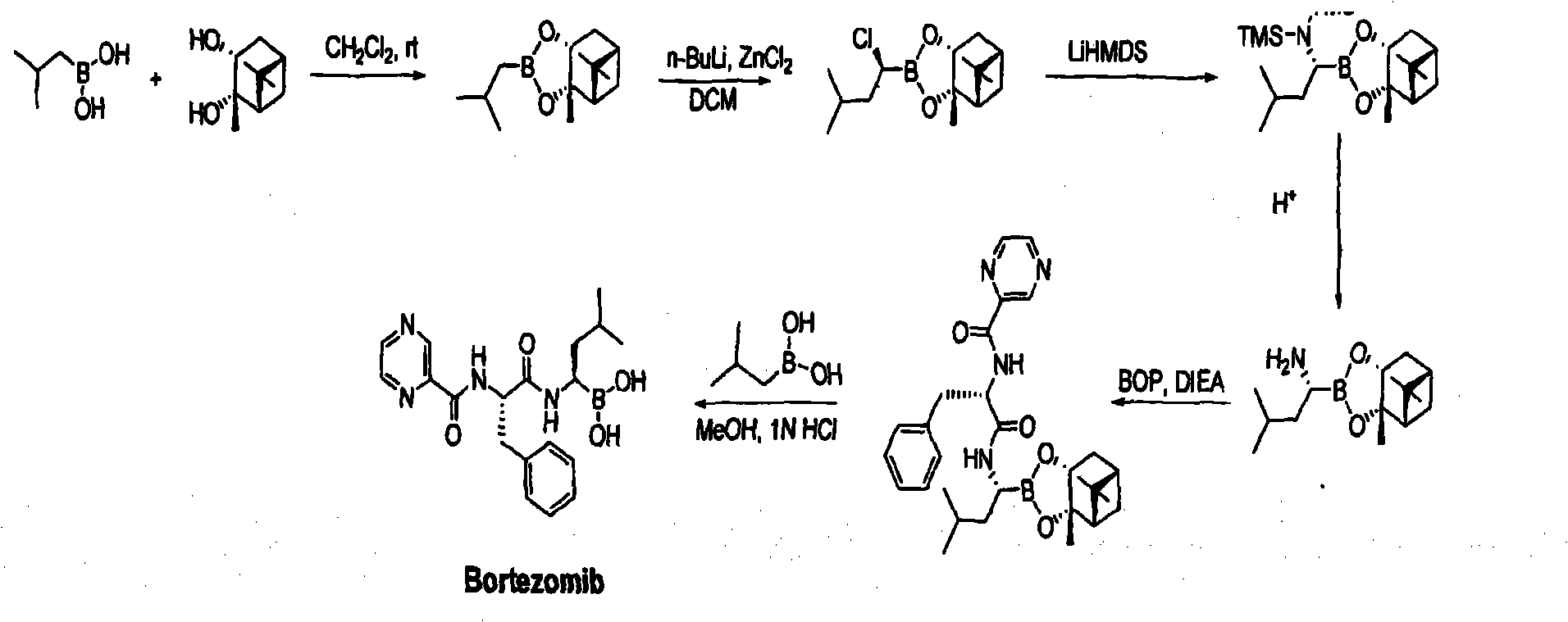
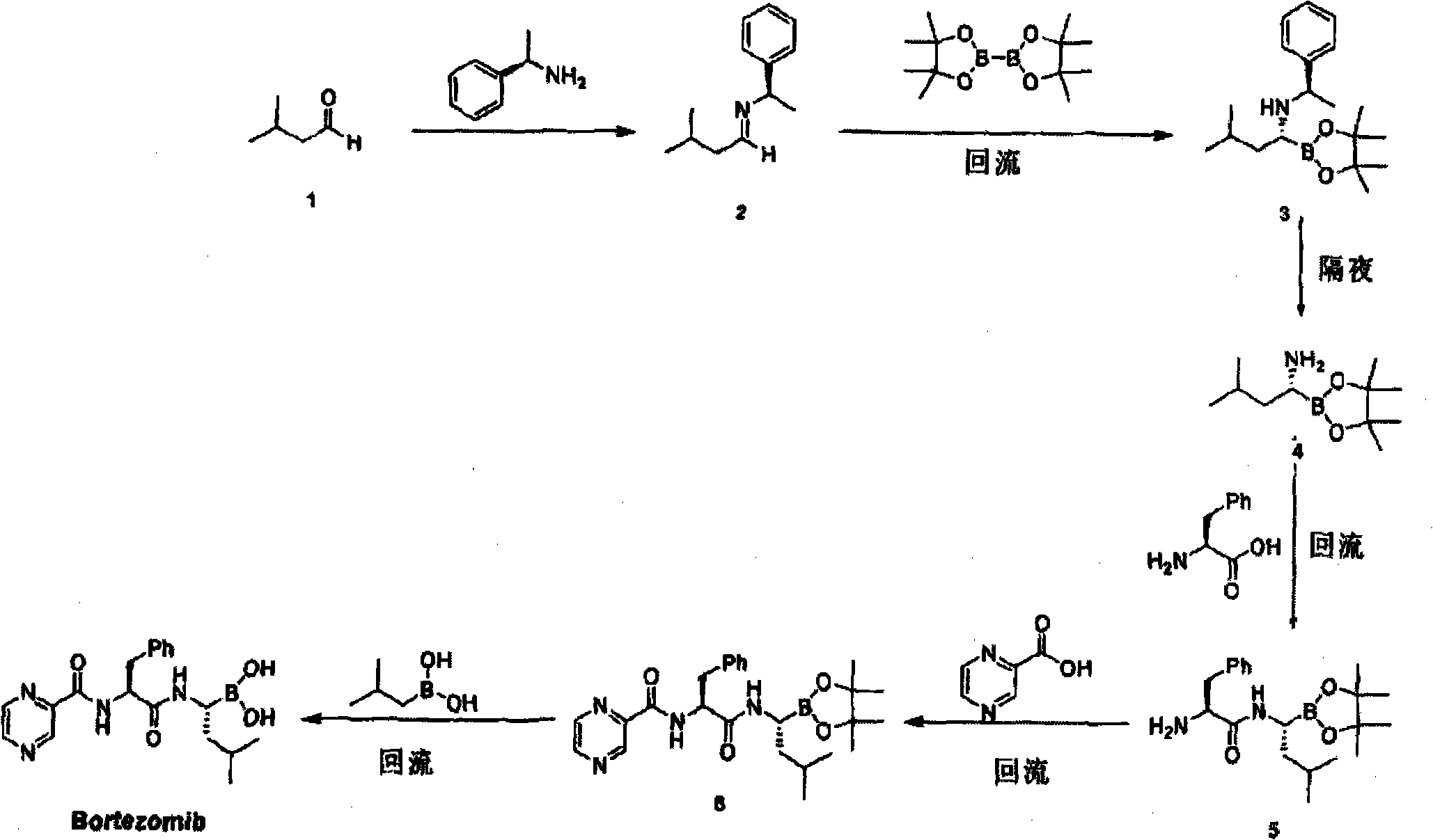
Improved method of bortezomib process
A bortezomib and process technology, applied in the field of pharmaceutical synthesis, can solve the problems of product purity, low yield, easy falling off of protective groups, easy decomposition of condensing agents, etc., achieving easy operation, inhibiting the generation of condensation by-products, and inhibiting products. The effect of racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 (αR)-(1S, 2S, 3R, 5S)-pinanediol-N-(N-trifluoroacetoxy-phenylpropylamino)-1-amino-3-methylbutane-1 - Preparation of boroesters
[0033] Add 5.4 g of N-trifluoroacetyl-L-phenylalanine into 55 ml of dichloromethane, stir and cool down to 8°C, add EDCI [1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride] 4.4g, (αR)-(1S, 2S, 3R, 5S)-pinanediol-1-amino-3-methylbutane-1-boronic acid trifluoroacetate 8.3g , add triethylamine dropwise to adjust the pH to 8. After the dropwise addition, keep warm for 2 hours, then rise to 25°C within 1 hour, keep warm for 2 hours, concentrate under reduced pressure, add 30ml of ethyl acetate to dissolve the oil, and add 20ml of water Washing, washing with 20ml of saturated sodium bicarbonate solution, washing with 20ml of water again, discarding the aqueous layer, drying the organic layer over anhydrous sodium sulfate, filtering, and concentrating under reduced pressure below 35°C to obtain compound V(αR)-(1S, 2S, 3R, 5S)...
Embodiment 2
[0034] Example 2 (αR)-(1S, 2S, 3R, 5S)-pinanediol-N-(N-trifluoroacetoxy-phenylpropylamino)-1-amino-3-methylbutane-1 - Preparation of boroesters
[0035] Add 5 g of N-trifluoroacetyl-L-phenylalanine into 60 ml of dichloromethane, stir and cool down to 10°C, add 8.9 g of TBTU+HOBT (molar ratio 1:1), (1S, 2S, 3R, 5S )-pinanediol L-phenylalanine-L-leucine boric acid trifluoroacetate 8.5g, add dropwise triethylamine to adjust the pH=9.6, after the dropwise addition is completed, keep the reaction for 2.5h, then in 1 hour Rise to 18°C, keep warm for 2.3 hours, concentrate under reduced pressure, add 30ml of ethyl acetate to dissolve the oil, wash with 45ml of water, 45ml of 3% citric acid aqueous solution, 45ml of saturated sodium bicarbonate solution, and wash again with 45ml of water , discard the aqueous layer, dry the ethyl acetate layer over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure at 35°C to obtain compound V (αR)-(1S, 2S, 3R, 5S)-...
Embodiment 3
[0036] Example 3 (αR)-(1S, 2S, 3R, 5S)-pinanediol-N-(N-trifluoroacetoxy-phenylpropylamino)-1-amino-3-methylbutane-1 - Preparation of boroesters
[0037] Add 5.3 g of N-trifluoroacetyl-L-phenylalanine into 65 ml of dichloromethane, stir and cool down to 5°C, add 6.8 g of EDCI+HOOBT (molar ratio 1:1.3), (1S, 2S, 3R, 5S)-pinanediol L-phenylalanine-L-leucine boric acid trifluoroacetate 8.5g, add dropwise triethylamine to adjust pH=10, after the dropwise addition is completed, keep warm for 1.8h, then in 1 Rise to 18°C within 1 hour, keep warm for 2.1h, concentrate under reduced pressure, add 30ml of ethyl acetate to dissolve the oil, wash with 40ml of water, 40ml of 3% citric acid aqueous solution, 40ml of saturated sodium bicarbonate solution, and again with 40ml of Wash with water, discard the aqueous layer, dry the ethyl acetate layer over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure at 35°C to obtain compound V (αR)-(1S, 2S, 3R, 5S)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com