Synthetic method for sartan drug intermediate and application of intermediate
A synthetic method and intermediate technology, applied in the field of pharmaceutical synthesis, can solve the problems of mediocre effect and high toxicity of organotin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
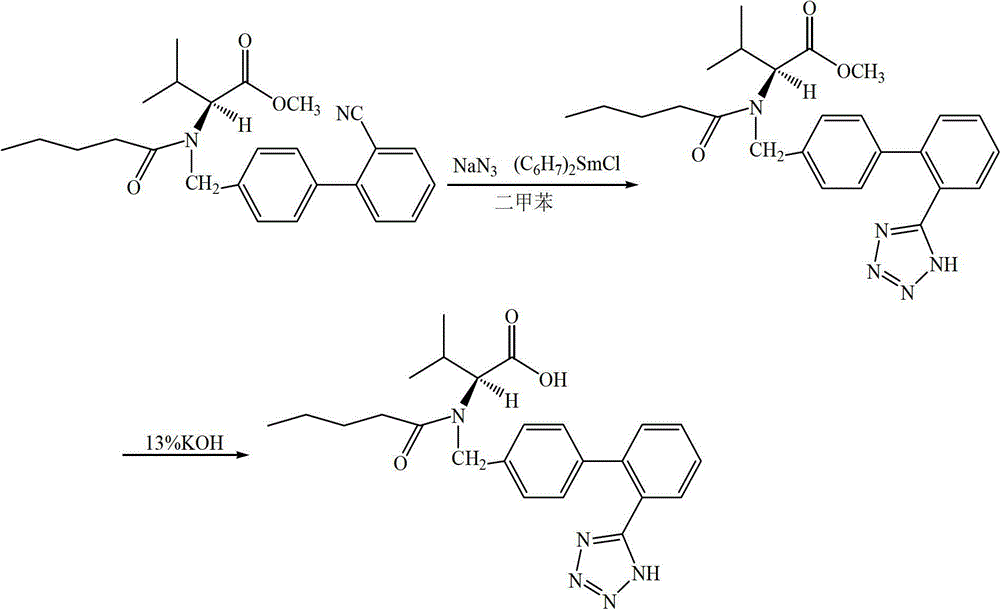
[0037] 40.6g (0.10mol) of N-[(2'-cyanobiphenyl-4-yl)methyl]-N-valeryl-(L)-valine methyl ester was dissolved in 400mL of dried xylene In addition to water and oxygen, and nitrogen protection, 37.8g (0.11mol) of bis(methylcyclopentadiene)samarium chloride and 7.2g (0.11mol) of sodium azide were added to the reaction solution, and the temperature was raised to 130℃ React until the reaction is complete (reaction time 1.8 hours). After the reaction was completed, the temperature was lowered to room temperature, 200 mL of purified water was added for washing and extraction, and the mixture was separated by standing. The organic layer was extracted twice with saturated brine, the organic phase was separated, 350 mL of 13% potassium hydroxide aqueous solution was added, and the temperature was raised to 40°C for reaction. After the reaction is over (reaction time 3 hours), the organic phase is separated and the organic phase is washed with 80 mL of 13% potassium hydroxide aqueous solu...
Embodiment 2
[0041] Dissolve 60.9g (0.15mol) of N-[(2'-cyanobiphenyl-4-yl)methyl]-N-valeryl-(L)-valine methyl ester in 600mL of dried xylene In addition to water and oxygen, protected by nitrogen, 56.8g (0.165mol) of bis(methylcyclopentadiene)samarium chloride and 10.8g (0.166mol) of sodium azide were added to the reaction solution, and the temperature was raised to 130°C React until the reaction is complete (reaction time 2 hours). After the reaction was completed, the temperature was lowered to room temperature, 300 mL of purified water was added for washing and extraction, and the mixture was separated by standing. The organic layer was extracted twice with saturated brine, the organic phase was separated, 530 mL of 13% potassium hydroxide aqueous solution was added, and the temperature was raised to 40° C. for reaction. After the completion of the reaction (reaction time 3.5 hours), the organic phase was allowed to stand and separate into layers. The organic phase was washed with 120 m...
Embodiment 3
[0045] Other conditions are the same as in Example 1, the difference is that in the synthesis of the intermediate: the organic solvent used is DMF; the molar ratio, the amount of bis(methylcyclopentadiene)samarium chloride is N-[(2′ -Cyanobiphenyl-4-yl)methyl]-N-valeryl-(L)-valine methyl ester 1.2 times; N-[(2′-cyanobiphenyl-4-yl)methyl The molar ratio of N-N-pentanoyl-(L)-valine methyl ester to sodium azide is 1:0.8; N-[(2′-cyanobiphenyl-4-yl)methyl] The ratio of -N-valeryl-(L)-valine methyl ester to organic solvent DMF is 1:10 (g:mL); the reaction temperature is 120°C, and the reaction time is 2 hours.
[0046] In the hydrolysis reaction for the synthesis of valsartan: the alkaline hydrolysis reagent is potassium hydroxide, the hydrolysis temperature is 50°C, and the volume (mL) of potassium hydroxide is N-[(2′-cyanobiphenyl-4-yl) 9 times the mass (g) of methyl]-N-valeryl-(L)-valine methyl ester, and the reaction time is 4 hours.
[0047] The yield of the crude valsartan is 90%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com