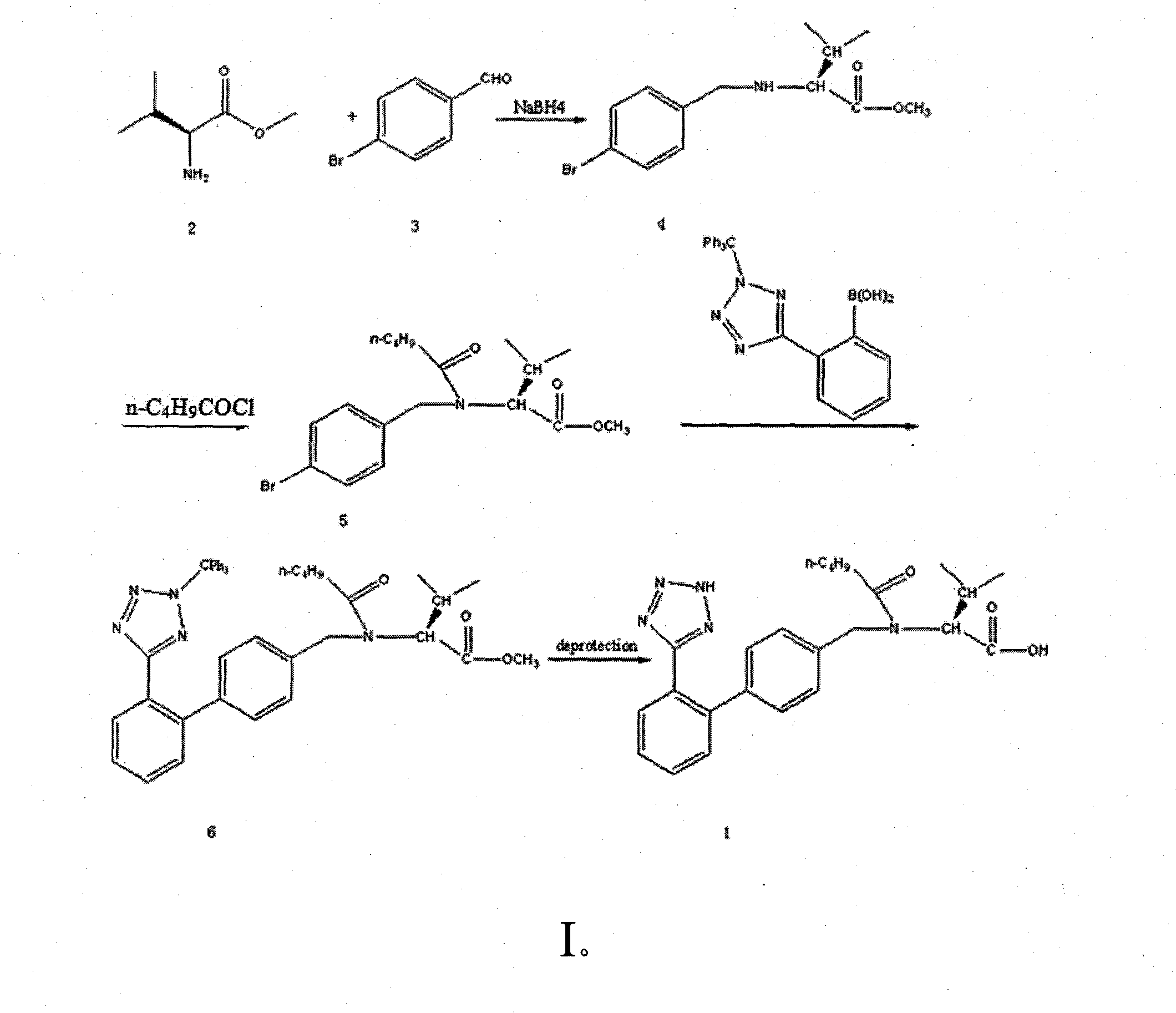
Preparation of Valsartan
A technology of valsartan and valine methyl ester, applied in the field of drug synthesis, to achieve the effect of safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] step a
[0021] Preparation of N-[(4-bromo)-benzylidene]-L-valine methyl ester
[0022] Add 16ml of triethylamine to 14g, 0.082mol of L-valine methyl ester hydrochloride in 120ml of tetrahydrofuran, then add 15g of anhydrous magnesium sulfate, and after fully stirring the reaction, add 9.7g, 0.064mol of p-bromobenzene Formaldehyde, after the reaction was complete, the two phases were separated, the liquid phase was evaporated in vacuo and dried in high vacuum to give a pale yellow oil. It was directly put into the next reaction without further treatment.
[0023] step b
[0024] Preparation of N-[(4-bromo)-benzyl]-L-valine methyl ester
[0025] The oil was dissolved in 150 ml of dry methanol. Cool the solution to 0°C, then slowly add sodium borohydride for one hour with stirring until the reaction of the imine is complete, add 40ml of water to the solution, evaporate the solution in a vacuum, add 700ml of water and 100ml of dihydrogen to the remaining liquid Chloro...
Embodiment 2
[0036] step a
[0037] Preparation of N-[(4-bromo)-benzylidene]-L-valine methyl ester
[0038] Add 16ml of triethylamine to 15g of L-valine tert-butyl ester hydrochloride, 0.082mol in 120ml of dichloromethane, then add 20g of anhydrous magnesium sulfate, after fully stirring the reaction, add 9.7g, 0.064mol of p-Bromobenzaldehyde, after the reaction is complete, the two phases are separated, the liquid phase is evaporated in vacuo and dried in high vacuum to give a pale yellow oil.
[0039] It was directly put into the next reaction without further treatment.
[0040] step b
[0041] Preparation of N-[(4-bromo)-benzyl]-L-valine tert-butyl ester
[0042] Dissolve the oil in 180ml of anhydrous methanol, cool the solution to 0°C, then slowly add sodium borohydride with stirring, and finish adding within one hour until the reaction of the imine is complete, add 40ml of water to the solution, and dissolve the solution Evaporate in vacuo, add 700ml of water and 100ml of dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com