Axial ligand modified homonuclear bimetallic compound catalyst and preparation method and application thereof
A compound and bimetal technology, applied to a homonuclear bimetallic compound catalyst and its preparation method and application field, can solve the problems of low selectivity and high price, and achieve the effects of fast reaction speed, avoiding synthesis steps and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The embodiment of the present invention provides a preparation method of an axial ligand-modified homonuclear bimetallic compound catalyst, which includes: comprising Rh 2 (esp) 2 A mixed system of the axial ligand and the organic solvent is reacted at room temperature to obtain an axial ligand-modified homonuclear bimetallic compound catalyst, wherein the axial ligand includes an organic ligand containing unsaturated nitrogen.
[0020] Specifically, the preparation method includes: to Rh 2 (esp) 2 The axial ligand is added to the organic solvent solution until the color of the solution changes from green to light red, and the homonuclear bimetallic compound catalyst modified by the axial ligand is obtained.
[0021] Further, the amount of the axial ligand is much greater than that of Rh 2 (esp) 2 dosage.
[0022] Further, the axial ligand and Rh 2 (esp) 2 The molar ratio is not less than 2:1.
[0023] Further, the organic ligand containing unsaturated nitrogen ...
Embodiment 1
[0038] Example 1: Axial Ligand Modified Rh 2 (esp) 2 Catalyst preparation:
[0039] Step 1: Prepare 3.8mg / mL Rh at room temperature 2 (esp) 2 dichloromethane solution;
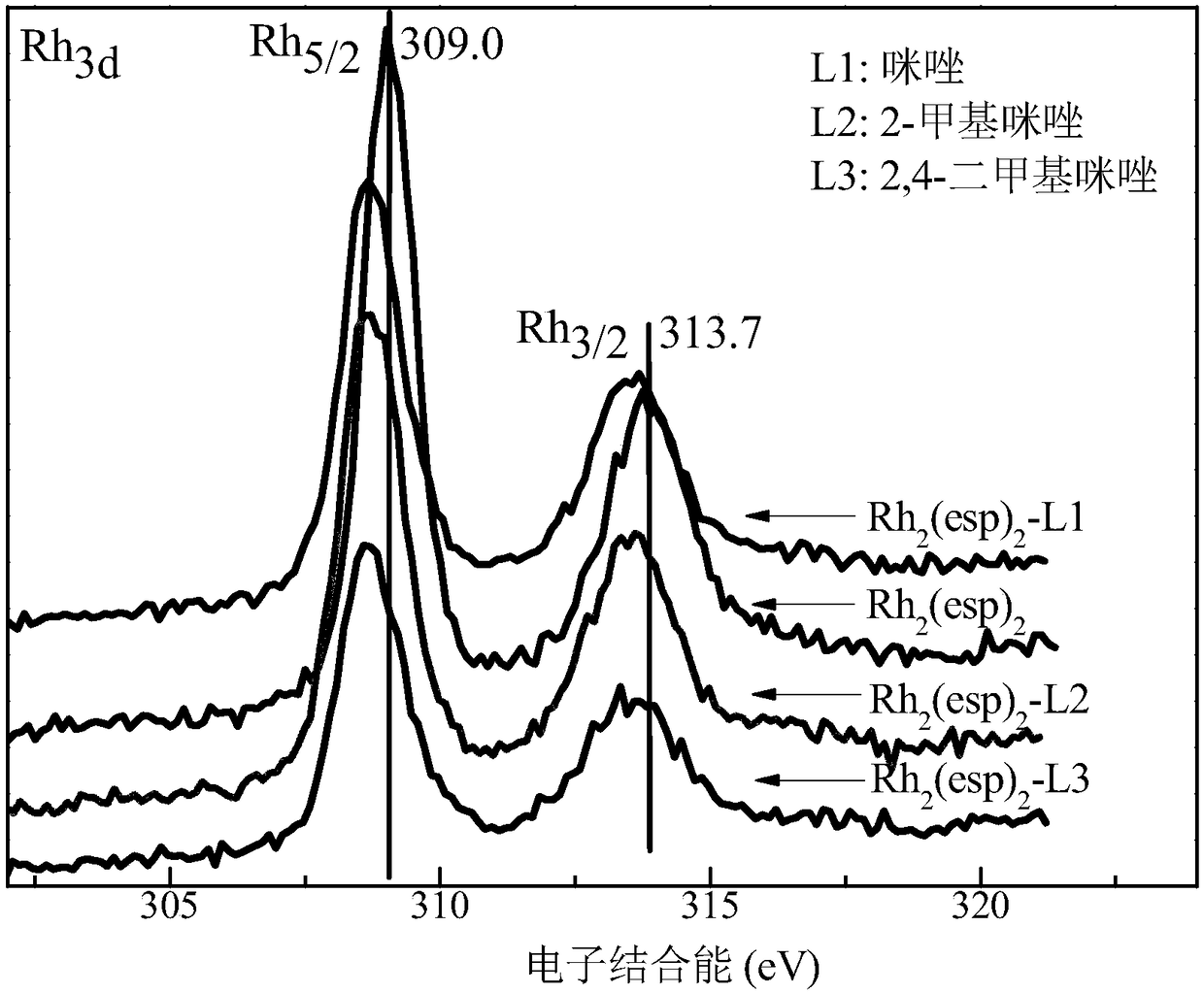
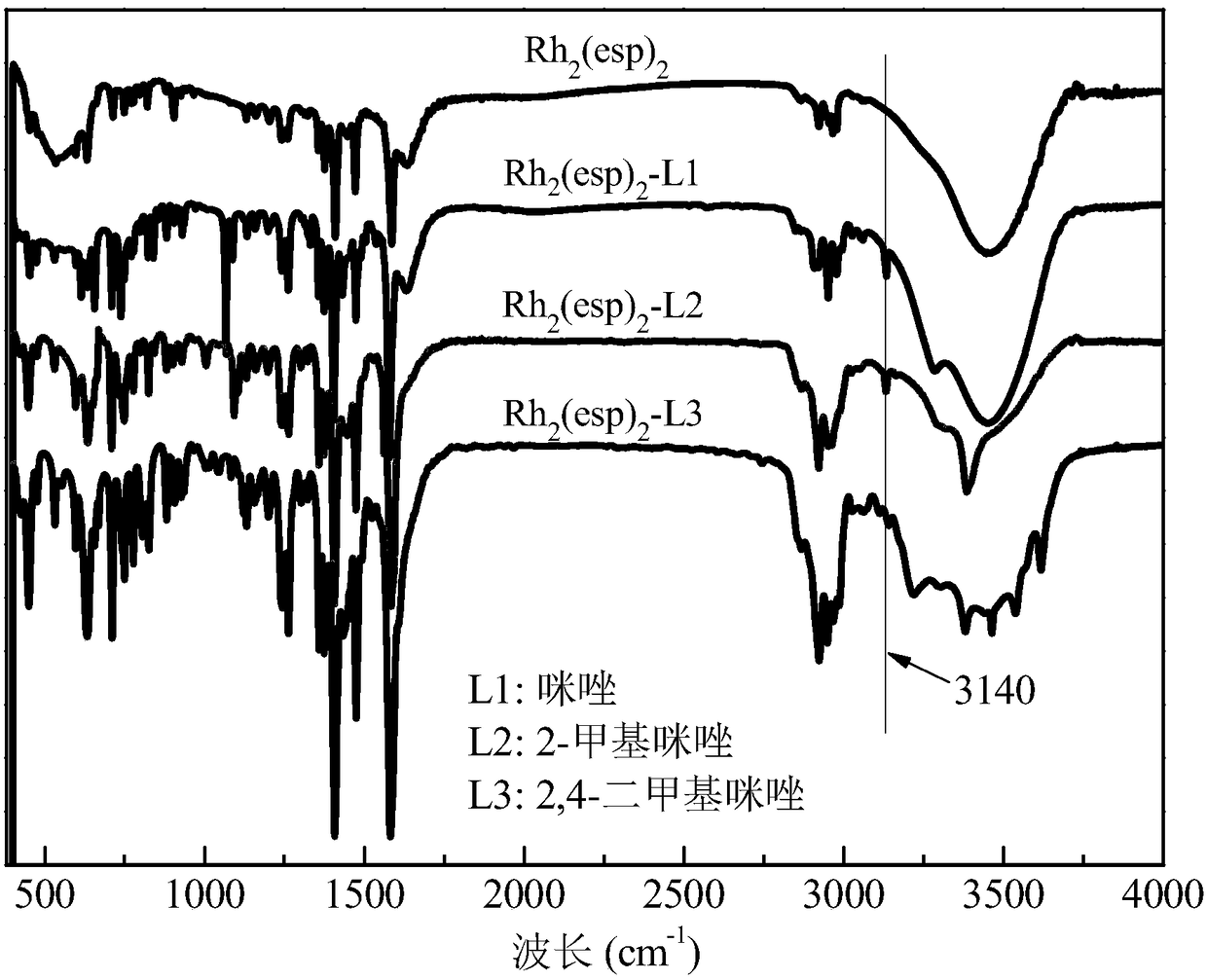
[0040] Step 2: Take 10mL of the above solution and gradually add different axial ligands (refer to Table 1) to it. Stir fully with a magnetic stirrer at room temperature, the color of the solution will gradually change from green to red, and the color of the solution will not change anymore It can be judged that the coordination is complete, the excess axial ligand is extracted, and the coordinated Rh 2 (esp) 2 The dichloromethane solution was suspended and evaporated to dryness to obtain the Rh after axial ligand modification (coordination) 2 (esp) 2 Catalyst, labeled Rh 2 (esp) 2 -L. Among them, Rh modified by axial ligands imidazole, 2-methylimidazole and 2,4-dimethylimidazole 2 (esp) 2 The X-ray photoelectron spectrum of figure 1 Shown; Rh modified by the axial ligands imidazole, 2-methylimida...
Embodiment 2
[0042] Embodiment 2: the Rh 2 (esp) 2 -L is applied to the process of catalyzing the synthesis of N-H heterocyclopropane compounds as follows:
[0043] The reaction was carried out in a 10mL reaction flask, and 0.55mmol of 2,4-dinitrophenylhydroxylamine, 0.005mmolRh 2 (esp) 2 - Add the reagent into the reaction bottle, vacuumize it with an oil pump and then fill it with nitrogen, and do this three times, then add 5.0mL of 2,2,2-trifluoroethanol solvent and 0.50mmol of 2-formazol with a syringe Base-1-phenylpropene, reacted at room temperature for 2h. Take 0.60 μL of the clear liquid of the reaction product, and quantitatively analyze the reaction product with a gas chromatograph, and the results are shown in Table 1:
[0044] Table 1 for different axial ligands and Rh 2 (esp) 2 Effect of Axial Coordination on Catalytic Performance
[0045]
[0046] Note: L is the axial ligand
[0047] The test results show that for the catalytic synthesis of N-H heterocyclopropane c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com