A kind of heterogeneous synthesis method and application of ritonavir intermediate
A technology for ritonavir and intermediates, which is applied in the field of pharmaceutical synthesis, can solve problems such as increasing production cost and technological difficulty, and achieves the effects of improving production safety, reducing harm and reducing water content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
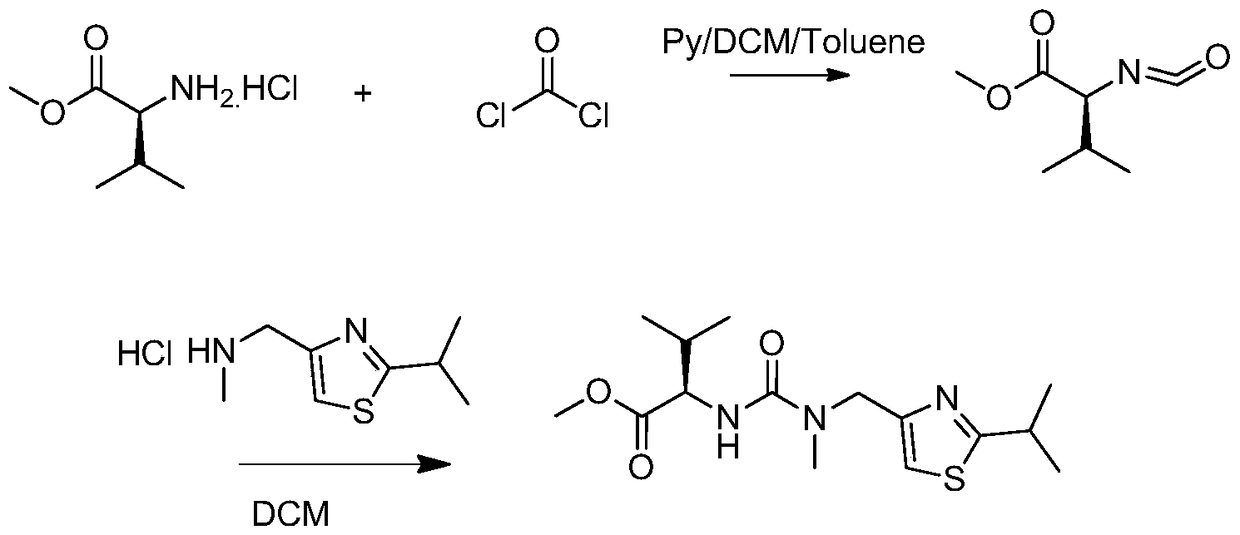
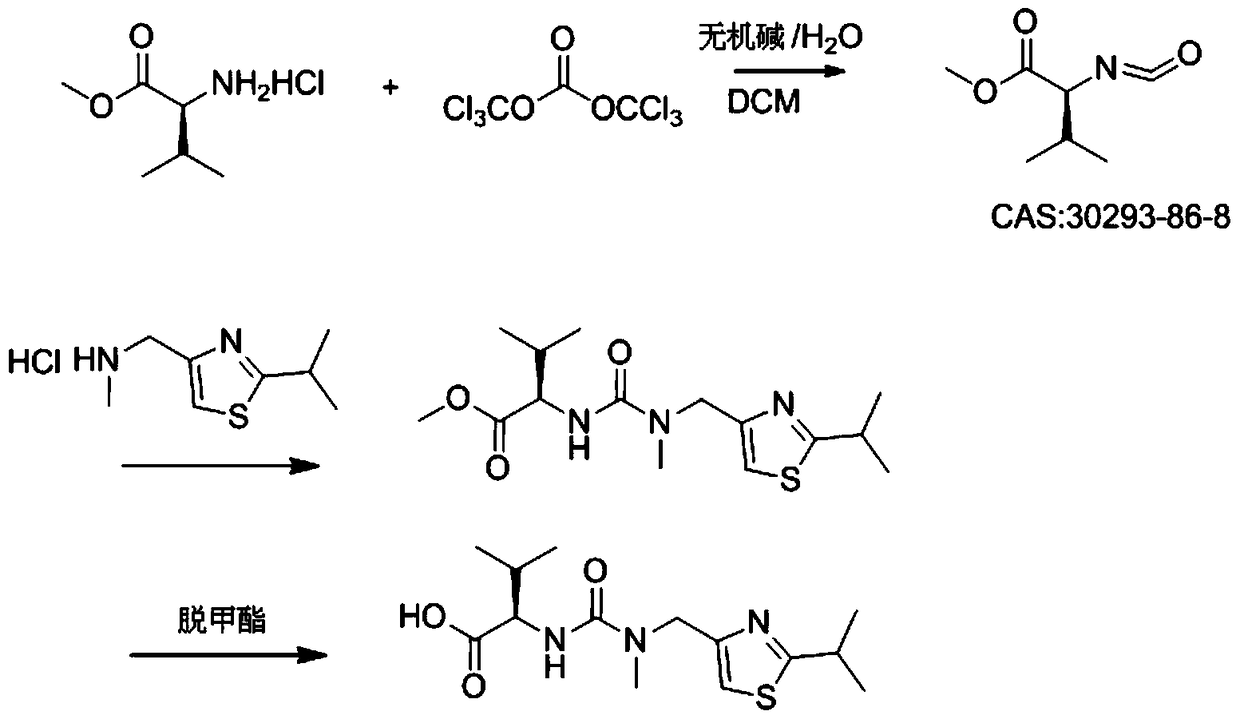
[0028] In a 1000ml four-necked bottle, dissolve 30g (0.18mol) of L-valine methyl ester hydrochloride and 18-21.2g of triphosgene in 270ml of dichloromethane, and add it dropwise under nitrogen protection at 5-10°C Add 480ml of 1.2M sodium bicarbonate aqueous solution (0.57mol) and finish adding in about 1h and 10min; then raise the temperature to 35-40°C, keep stirring for 1h to react and generate isocyanate compound (S)-(-)-2-isocyanate Acyl-3-methylbutyric acid methyl ester; Then add 32g of 2-isopropyl-4-(methylaminomethyl)thiazole hydrochloride in batches at 35~40°C, during the addition process use 50g of 10% sodium hydroxide solution was used to adjust the pH value to be stable at 6; after the dropwise addition was completed, the temperature was kept at 35-40°C for 1 hour; after the reaction was monitored by HPLC, the liquid was separated, and the aqueous phase was extracted with 100g of dichloromethane, and the extract was mixed with the organic phase Combined, then washe...
Embodiment 2
[0033] In a 1000ml four-necked bottle, dissolve 30g (0.18mol) of L-valine methyl ester hydrochloride and 18-21.2g of triphosgene in 270ml of dichloromethane, and add it dropwise under nitrogen protection at 10-20°C Add 480ml of 1.2M sodium bicarbonate aqueous solution (0.57mol) and finish adding in about 1 hour; then raise the temperature to 30°C, keep stirring for 1 hour to react to generate isocyanate compound (S)-(-)-2-isocyanato-3 - methyl methyl butyrate; then add 32 g of 2-isopropyl-4- (methylaminomethyl) thiazole hydrochloride in batches at 30°C, use 50 g of 10% hydrogen during the addition The sodium oxide solution was used to adjust the pH value to be stable at 6; after the dropwise addition was completed, the temperature was kept at 30°C for 1 hour; after the reaction was monitored by HPLC, the liquid was separated, and the aqueous phase was extracted with 100g of dichloromethane, the extract was combined with the organic phase, and then 200g of 15 Wash once with % s...
Embodiment 3
[0035] In a 1000ml four-neck flask, dissolve 30g (0.18mol) of L-valine methyl ester hydrochloride and 21g of triphosgene in 270ml of dichloromethane, and add 480ml of it dropwise under nitrogen protection at 20-28°C It is 1.2M sodium bicarbonate aqueous solution (0.57mol), about 1h to add; then keep stirring for 1h to react to generate isocyanate compound (S)-(-)-2-isocyanato-3-methylbutyric acid methyl ester; Then add 32g of 2-isopropyl-4-(methylaminomethyl)thiazole hydrochloride in batches at 20~28°C, adjust the pH value with 10% sodium hydroxide solution of 50g in the process of adding Stable at 6; after the dropwise addition, keep warm at 20-28°C for 1 hour; after the reaction is monitored by HPLC, separate the liquids, extract the aqueous phase with 100g of dichloromethane, combine the extract with the organic phase, and then use 200g of 15% sodium chloride The solution was washed once; the liquid was separated, and the organic phase was evaporated to dryness under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com