Ph-responsive polyaspartic acid grafted with hydrophobic amino acid and preparation method thereof
A technology of hydrophobic amino acid and polyaspartic acid is applied in the field of biomedical drug carrier materials, which can solve the problems of unseen continuous grafting of two hydrophobic amino acids, and achieves good cell compatibility, easy availability of experimental materials, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
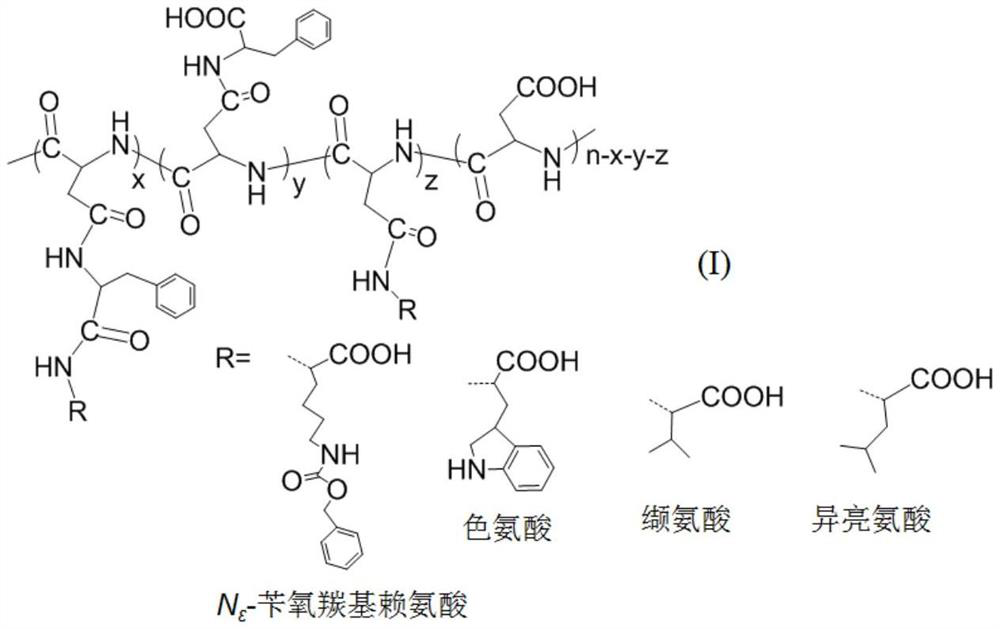
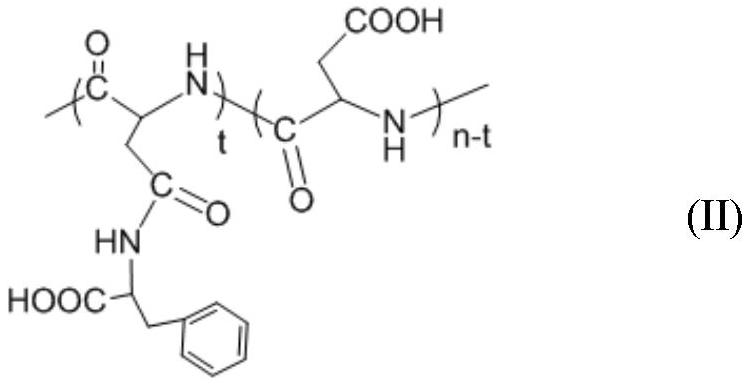
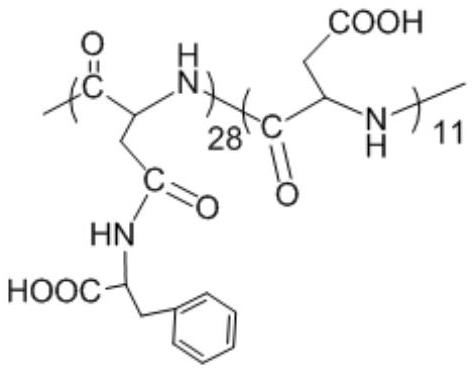
[0030] (1) Preparation of polyaspartic acid-graft-phenylalanine-graft-benzyloxycarbonyl lysine
[0031] 1) polyaspartic acid (molecular weight M w =4500) (3.00g, the amount of carboxyl substance is 0.026mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide 4.98g, 0.026mol) is dissolved in 150mL mass concentration is 0.63 In wt% aqueous sodium bicarbonate solution, a solution having a polyaspartic acid concentration of 2.0 wt% was obtained and stirred for 30 min;
[0032] 2) According to the molar ratio of polyaspartic acid carboxyl group and L-phenylalanine methyl ester hydrochloride 1:1.2, add L-phenylalanine methyl ester hydrochloride (6.69g, 0.031mol) aqueous solution and react at room temperature 72h;
[0033] 3) Add 75 mL of 5.0 wt% sodium hydroxide ethanol solution for alkali washing, and stir overnight at room temperature;
[0034] 4) The solution is packed into a dialysis bag with a molecular weight cut-off of 0.5kDa, dialyzed with deionized water for 3 days, purif...
Embodiment 2
[0069] (1) Preparation of polyaspartic acid-graft-phenylalanine-graft-benzyloxycarbonyl lysine
[0070] 1) polyaspartic acid (molecular weight M w =46100) (1.50g, the amount of carboxyl substance is 0.013mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide 4.98g, 0.026mol) dissolved in 150mL mass concentration is 0.21 wt% aqueous sodium bicarbonate solution to obtain a solution with a polyaspartic acid concentration of 1.0 wt%, and stir for 60 min;
[0071] 2) According to the molar ratio of polyaspartic acid carboxyl group and L-phenylalanine methyl ester hydrochloride 1:0.6, add L-phenylalanine methyl ester hydrochloride (1.68g, 0.0078mol) aqueous solution and react at room temperature 72h;
[0072] 3) Add 80 mL of 2.0 wt% sodium hydroxide ethanol solution for alkali washing, and stir overnight at room temperature;
[0073] 4) Put the solution into a dialysis bag with a molecular weight cut-off of 1 kDa, dialyze with deionized water for 3 days, purify, and freeze-dry fo...
Embodiment 3
[0108] (1) Preparation of polyaspartic acid-graft-phenylalanine-graft-benzyloxycarbonyl lysine
[0109] 1) polyaspartic acid (molecular weight M w =16800) (2.0g, the amount of carboxyl substance is 0.017mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide 2.3g, 0.012mol) are dissolved in 100mL mass concentration is 0.54 wt% aqueous sodium bicarbonate solution to obtain a solution with a polyaspartic acid concentration of 2.0 wt%, and stir for 50 min;
[0110] 2) According to the molar ratio of polyaspartic acid carboxyl group and L-phenylalanine methyl ester hydrochloride 1:0.9, add L-phenylalanine methyl ester hydrochloride (3.33g, 0.015mol) aqueous solution and react at room temperature 72h;
[0111] 3) Add 50 mL of 4.0 wt% sodium hydroxide ethanol solution for alkali washing, and stir overnight at room temperature;
[0112] 4) Put the solution into a dialysis bag with a molecular weight cut-off of 1 kDa, perform dialysis for 3 days, purify, freeze-dry for 24 hours to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com