Synthetic method of valsartan
A synthetic method, the technology of valsartan, which is applied in the field of valsartan synthesis, can solve the problems of low yield and high manufacturing cost, and achieve the effects of high yield, thorough reaction and sufficient contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
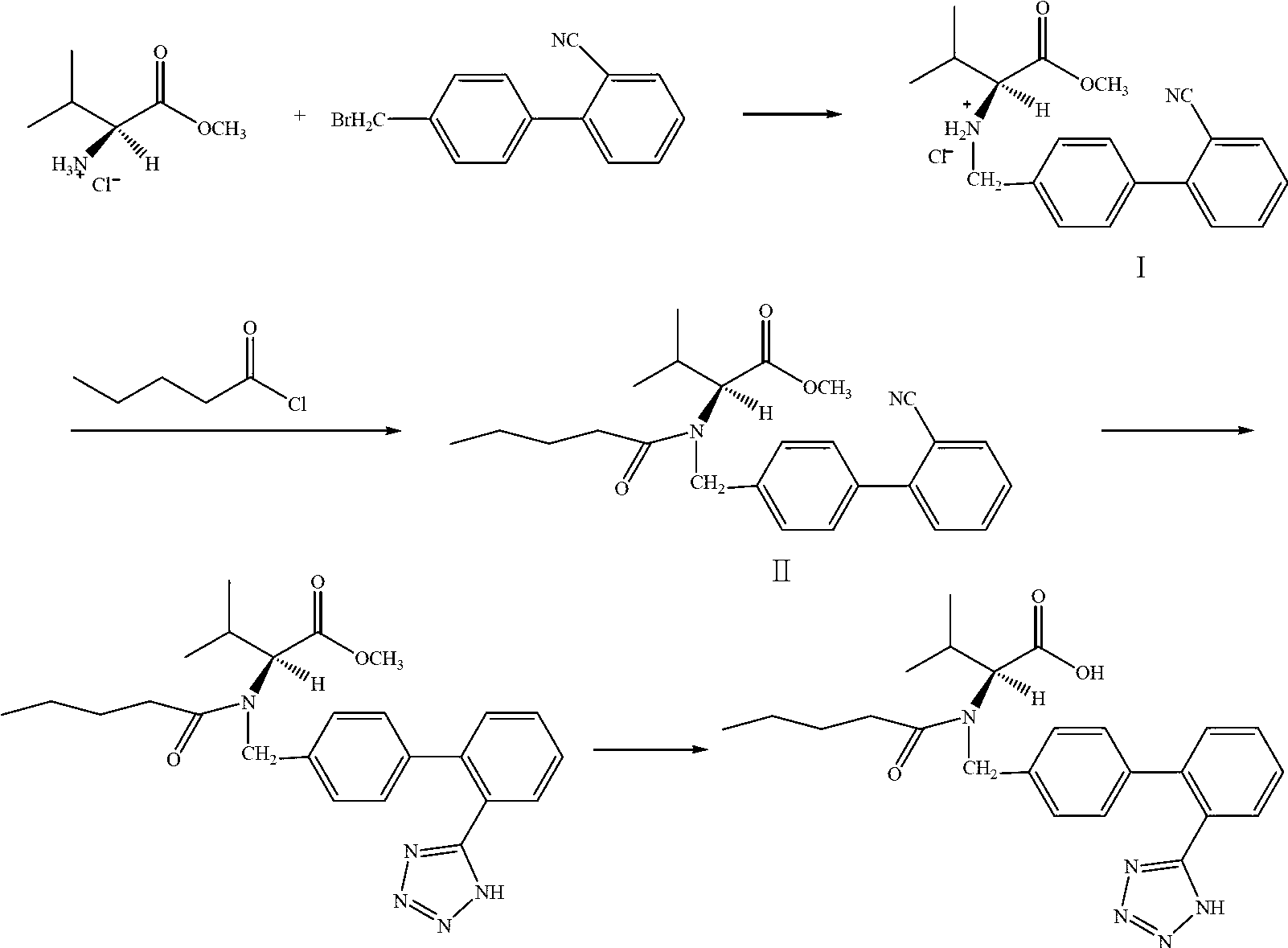
[0025] (1) Add 200ml purified water, 60g potassium carbonate, 42g L-valine methyl ester hydrochloride to the reaction flask, stir until dissolved, then add 500ml ethyl acetate and 95g 4-bromomethyl-2′- Add 2.3g of 18-crown-6 to cyanobiphenyl, stir, and heat to about 60°C for reflux reaction. After 2 hours of reaction, the reaction is over, the temperature is reduced to room temperature, the layers are separated, the organic layer is washed twice with 50ml of saturated brine. After washing, the organic phase is evaporated under reduced pressure, 250ml of purified water is added, stirred, and hydrochloric acid is added dropwise to pH =1-2, precipitated crystals, filtered with suction, washed, and dried to obtain 82.7g of intermediate I, with a yield of 92%.
[0026] (2) Add 240ml purified water and 114g potassium carbonate to the reaction flask and stir to clear. Add 620ml xylene, 2.7g 18-crown-6 and 82.7g Intermediate I and stir. At room temperature, drop a mixture of 34 g of n-...
Embodiment 2
[0031] (1) Add 200ml purified water, 60g potassium carbonate, 42g L-valine methyl ester hydrochloride to the reaction flask, stir until dissolved, add 500ml ethyl acetate and 95g 4-bromomethyl-2′-cyanide Then add 1.7g of 12-crown-4, stir, heat to about 60°C and reflux for reaction. After 2.5 hours of reaction, the reaction is over, the temperature is reduced to room temperature, the layers are separated, the organic layer is washed twice with 50ml of saturated brine, after washing, the organic phase is evaporated under reduced pressure, 250ml of purified water is added, stirring, and hydrochloric acid is added dropwise to pH =1-2, precipitated crystals, filtered with suction, washed, and dried to obtain 80.0g of intermediate I, with a yield of 89%.
[0032] (2) Add 240ml purified water and 110g potassium carbonate to the reaction flask and stir to clear. Then add 620ml xylene, 1.8g 12-crown-4 and 80g intermediate I and stir. At room temperature, drop a mixture of 33 g of n-vale...
Embodiment 3
[0037] (1) Add 200ml purified water, 60g potassium carbonate, 42g L-valine methyl ester hydrochloride to the reaction flask, stir until dissolved, then add 500ml ethyl acetate and 95g 4-bromomethyl-2′- To cyanobiphenyl, add 3.2g [2.2.2]-cryptone, stir, and heat to about 60°C for reflux reaction. After 3 hours of reaction, the reaction was over, the temperature was reduced to room temperature, the layers were separated, the organic layer was washed twice with 50ml of saturated brine. After washing, the organic phase was evaporated under reduced pressure, 250ml of purified water was added, stirred, and hydrochloric acid was added dropwise to pH =1-2, precipitated crystals, filtered with suction, washed, and dried to obtain 81.7g of intermediate I with a yield of 91%.
[0038] (2) Add 240ml purified water and 113g potassium carbonate to the reaction flask and stir to clear. Add 620ml xylene, 3.8g [2.2.2]-cryptone and 81.7g Intermediate I and stir. At room temperature, drop a mixtu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com