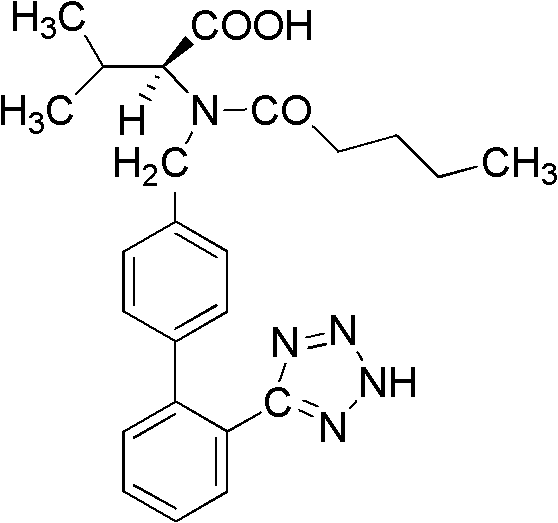
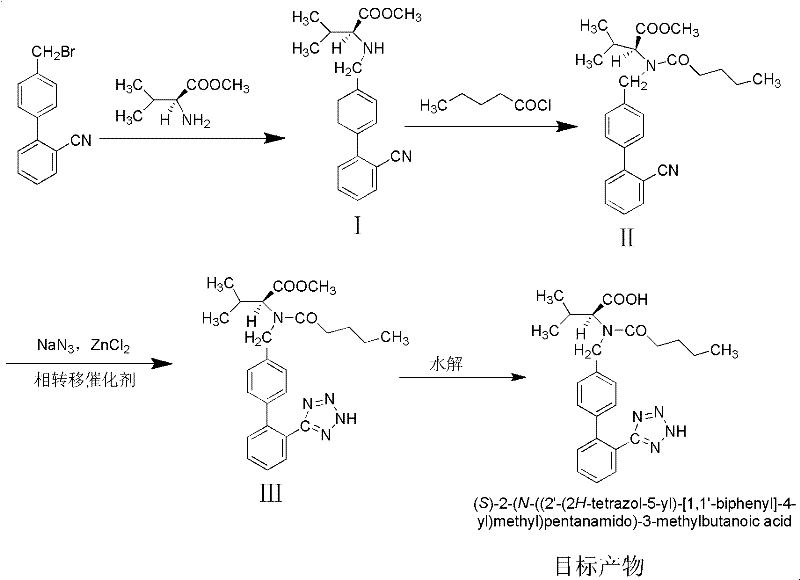
Method for synthesizing valsartan
A synthesis method and a technology for valsartan, applied in the field of preparation of antihypertensive drugs, can solve the problems of troublesome purification process, reduced yield, low yield and the like, and achieve the improvement of total yield, reduction of consumption of raw materials, and reduction of pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 1. Preparation of intermediate I N-(2'-cyanobiphenyl-4-methylene)-L-valine methyl ester hydrochloride
[0030] 1.1 Weigh 15g K 2 CO 3 Put it into a 250mL three-neck flask, add 100mL dichloroethane as a solvent, then weigh 13.2g (0.1mol) of L-valine methyl ester hydrochloride and 27.2g (0.1mol) of 4′-bromomethyl-2 - Add cyanobiphenyl to the three-necked flask, and add 1.7g (0.01mol) CuCl 2 and 2.0g (0.01mol) FeCl 2 As a catalyst, control the reaction temperature at 30-35°C, stir and react for 24 hours, after the reaction is complete, add 40 mL of deionized water and stir for 5 minutes, separate the mixed reaction liquid, keep the organic phase, and extract the water phase with 20 mL of dichloroethane, Combine the organic phases, wash with 30mL deionized water, remove the solvent by rotary evaporation, add 60mL dichloroethane, stir until the above product is completely dissolved, cool the temperature to 0-5°C, add 5mL concentrated hydrochloric acid, stir for 1h, and so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com