Benzimidazole derivative and use as angiotensin ii antagonist
A benzimidazole and biphenyl technology, applied in the field of new benzimidazole derivatives, can solve the problems of compound synthesis and separation unknown, reduce absorbability, reduce compound solubility, etc., and achieve excellent preventive or therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] 2-cyclopropyl-1-{[2'-(5-oxo-4,5-dihydro-1,2,4- Oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylic acid [(5-methyl-2-oxo-1,3-dioxolane En-4-yl)methyl]ester
[0197]
[0198] The compound (4.20 g) obtained in Reference Example 3 was dissolved in THF (42 ml), triethylamine (1.42 ml) and 2,4,6-trichlorobenzoyl chloride (1.52 ml) were added, and the mixture was stirred 12 hours. Insoluble materials were removed by filtration, and the filtrate was concentrated. The residue was dissolved in dichloromethane (42ml), medoxomil alcohol (1.45g) and DMAP (1.36g) were added, and the mixture was stirred for 12 hours. The reaction mixture was diluted with chloroform, washed successively with 1N hydrochloric acid, saturated aqueous sodium bicarbonate solution and saturated brine. The organic layer was dried over magnesium sulfate and concentrated to give the title compound (3.08 g, 59%).
[0199] 1H NMR (300MHz, DMSO-d 6 )δppm 0.97-1.10(m, 4H), 2.14(s, 3H), 2.1...
Embodiment 2
[0201] 3-{4'-[(2-cyclopropyl-7-{[(5-methyl-2-oxo-1,3-dioxol-4-yl)methoxy]carbonyl }-1H-benzimidazol-1-yl)methyl]biphenyl-2-yl}-1,2,4- Oxadiazole-5-potassium salt
[0202]
[0203] The compound obtained in Example 1 (1.00 g) was dissolved in acetone (20 ml), potassium 2-ethylhexanoate (0.323 g) was added, and the mixture was stirred for 4 hours and 30 minutes. Precipitated crystals were collected by filtration to obtain the title compound (0.581 g, 54%).
[0204] 1H NMR (300MHz, DMSO-d 6 )δppm 1.08(d, 4H, J=6.2Hz), 2.15(s, 3H), 2.25-2.34(m, 1H), 5.09(s, 2H), 5.84(s, 2H), 6.82(d, 2H, J=8.3Hz), 7.18-7.28(m, 4H), 7.29-7.42(m, 2H), 7.45-7.50(m, 1H), 7.53(dd, 1H, J=7.5, 1.1Hz), 7.80(dd , 1H, J=7.9, 1.1Hz).
Embodiment 3
[0206] 2-cyclopropyl-1-{[2'-(5-oxo-4,5-dihydro-1,2,4- Oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylic acid [(5-methyl-2-oxo-1,3-dioxolane Crystals of en-4-yl)methyl]ester
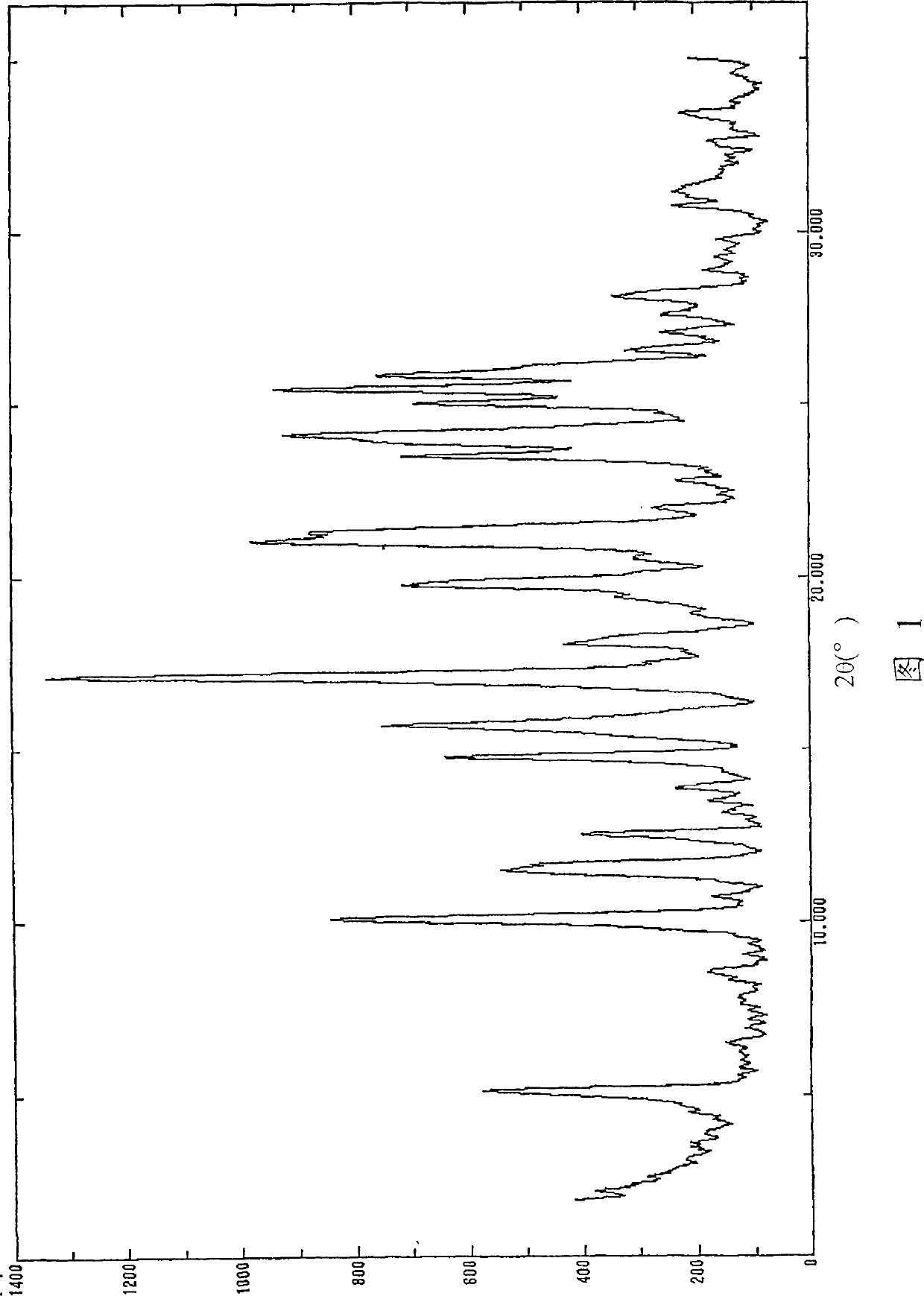
[0207] The compound obtained in Example 1 was recrystallized from acetonitrile to obtain solvate crystals containing acetonitrile. It was dried at 100° C. overnight under reduced pressure to obtain Form A crystals, which were thermally stable and practical. The obtained crystals had the powder X-ray crystal diffraction pattern shown in Fig. 1 and diffraction angles approximately as described below.
[0208] Table 1
[0209]
[0210]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com