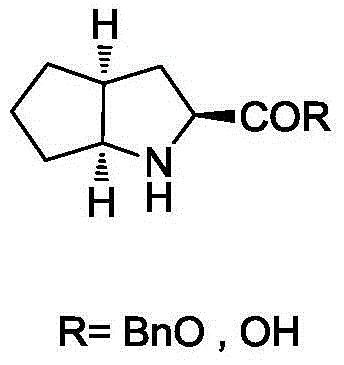
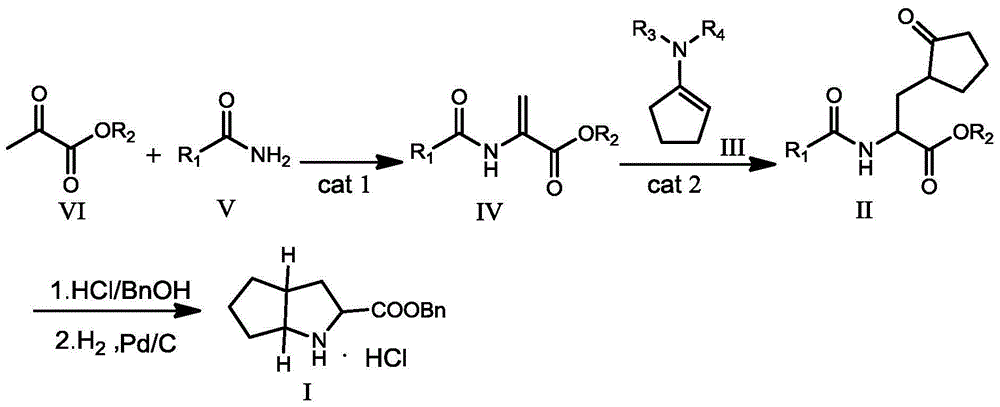
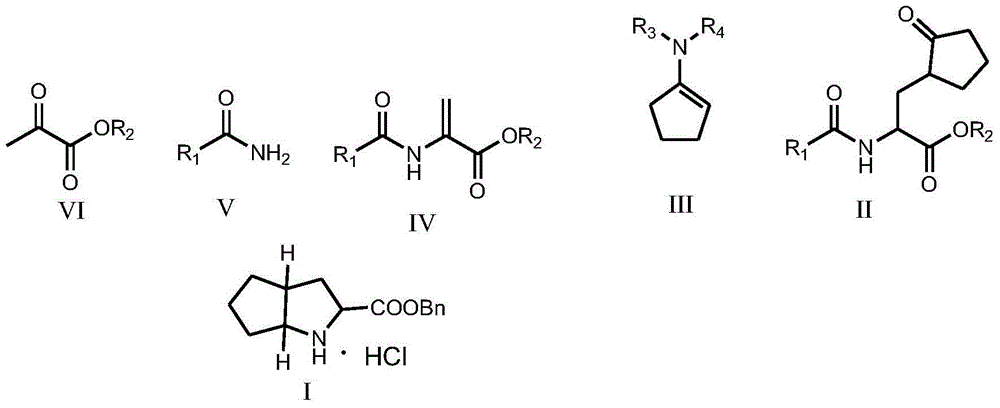
Ramipril intermediate synthesis method
A synthesis method and technology for intermediates, applied in the field of pharmaceutical chemical intermediate synthesis, can solve the problems of high pressure resistance, harsh reaction conditions, harsh hydrogenation conditions, etc., and achieve easy separation and purification, short reaction period and few synthesis steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Preparation of ethyl 2-acetamidoacrylate (IV-1)
[0053] Add 5.9g (0.10mol) of acetamide, 9.28g (0.08mol) of ethyl pyruvate, 0.138g (0.003mol) of formic acid, and 150mL of acetonitrile into a 500mL three-necked flask equipped with mechanical stirring and a thermometer, stir and heat to 80°C , TLC detected that the reaction was completed after 24 hours of reaction, the solvent was distilled off, cooled and left standing, and dichloromethane was added to dissolve the residue, washed with water, and the organic layer was dried and concentrated to obtain 6.91 g of compound 2-acetamido ethyl acrylate (IV-1). Rate 55.0%.
Embodiment 2
[0054] Embodiment 2: Preparation of ethyl 2-acetamidoacrylate (IV-1)
[0055] Add 5.9g (0.10mol) of acetamide, 17.4g (0.15mol) of ethyl pyruvate, 2.28g (0.02mol) of trifluoroacetic acid, and 150mL of ethanol into a 500mL three-necked flask equipped with mechanical stirring and a thermometer, stir and heat to 60°C, TLC detection, reacted for 26h, after the reaction, distilled off the solvent, cooled and stood still, added dichloromethane to dissolve the residue, washed with water, took the organic layer to dry, concentrated to obtain the compound 2-acetamido ethyl acrylate (IV-1 ) 8.54g, yield 54.4%.
Embodiment 3
[0056] Embodiment 3: Preparation of ethyl 2-acetamidoacrylate (IV-1)
[0057] Add 5.9g (0.10mol) of acetamide, 23.2g (0.20mol) of ethyl pyruvate, 3.19g (0.01mol) of trifluoromethanesulfonate di Aniline salt, 200mL toluene, stirred and heated to reflux state at 110°C, detected by TLC, reacted for about 16h, after the reaction, distilled off the solvent, cooled and stood still, added dichloromethane to dissolve the residue, washed with water, dried the organic layer, concentrated, After purification by column chromatography, 9.11 g of ethyl 2-acetamidoacrylate (IV-1) was obtained, with a yield of 58.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com