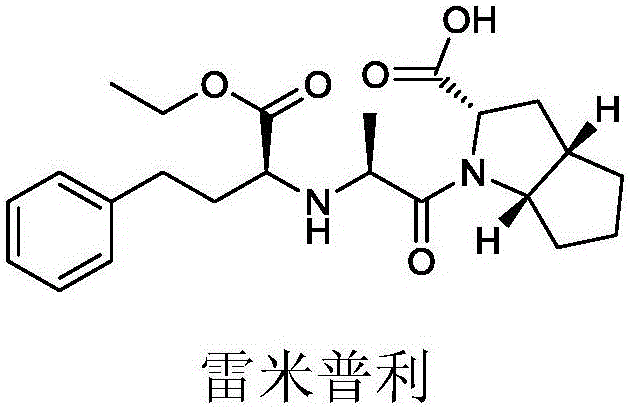
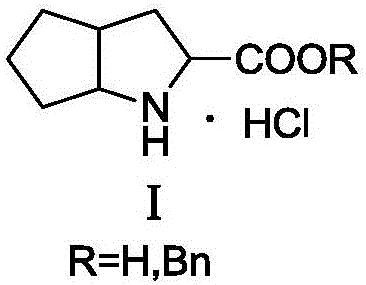
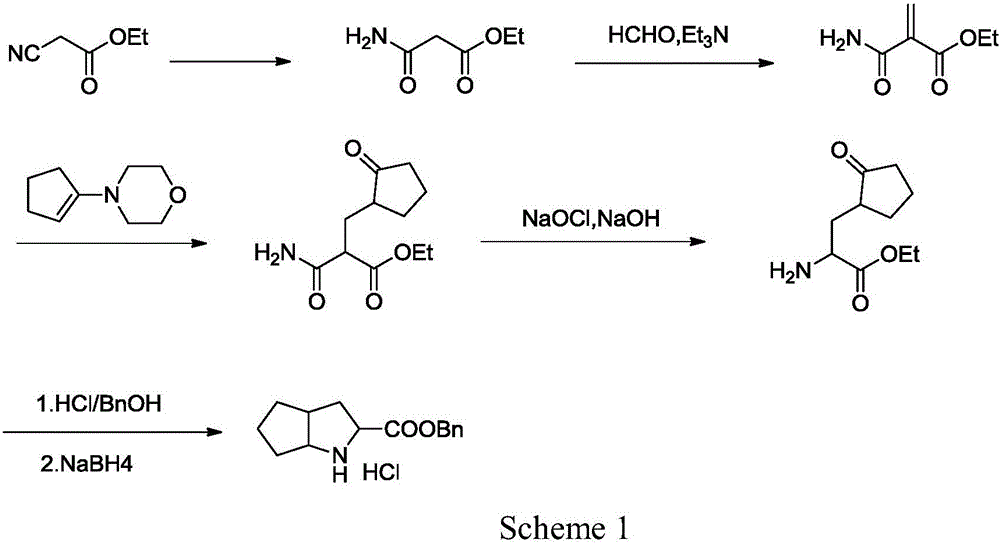
Synthesizing method using serine to prepare Ramipril key intermediate
A synthesis method and a technology for intermediates, which are applied in the field of synthesis of pharmaceutical and chemical intermediates, can solve the problems of low yield, unfavorable industrial production, large amount of concentrated sulfuric acid, expensive raw materials, etc., and achieve low-cost raw materials, avoid heavy metal pollution, and reduce three wastes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1, DL-serine methyl ester hydrochloride (Ⅲ)
[0047] Add 30mL of methanol into a 100mL three-necked flask, and slowly add 5.4mL of thionyl chloride dropwise under the condition of mechanical stirring in an ice-water bath. , heated to reflux for reaction, and after a reaction time of 20 h, the solvent was distilled off under reduced pressure, and 8.28 g of DL-serine methyl ester hydrochloride (Ⅲ) was obtained after drying, with a yield of 93.7%.
Embodiment 2
[0048] The preparation of embodiment 2, DL-serine methyl ester hydrochloride (Ⅲ)
[0049] Add 30mL of methanol into a 100mL three-necked flask, and slowly add 6.6mL of thionyl chloride dropwise under the condition of mechanical stirring in an ice-water bath. After the dropwise addition, continue to stir for 1h, and put 6.00g (57mmol) of DL-serine (II) into the three-necked flask , heated to reflux for reaction, and after a reaction time of 18 hours, the solvent was distilled off under reduced pressure and dried to obtain 8.32 g of DL-serine methyl ester hydrochloride (Ⅲ), with a yield of 94.2%.
Embodiment 3
[0050] The preparation of embodiment 3, L-serine methyl ester hydrochloride (Ⅲ)
[0051] Add 30mL of methanol into a 100mL three-necked flask, and slowly add 5.4mL of thionyl chloride dropwise under the condition of mechanical stirring in an ice-water bath. , heated to reflux for reaction, and after a reaction time of 10 h, the solvent was distilled off under reduced pressure to obtain 8.46 g of L-serine methyl ester hydrochloride (Ⅲ), with a yield of 95.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com