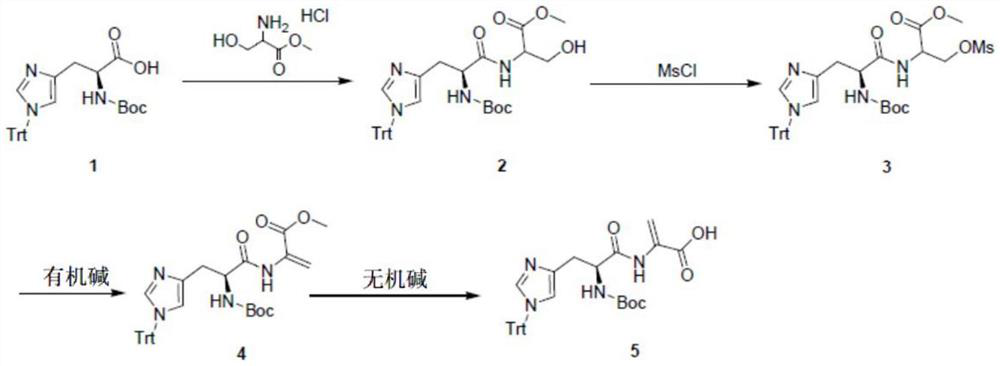
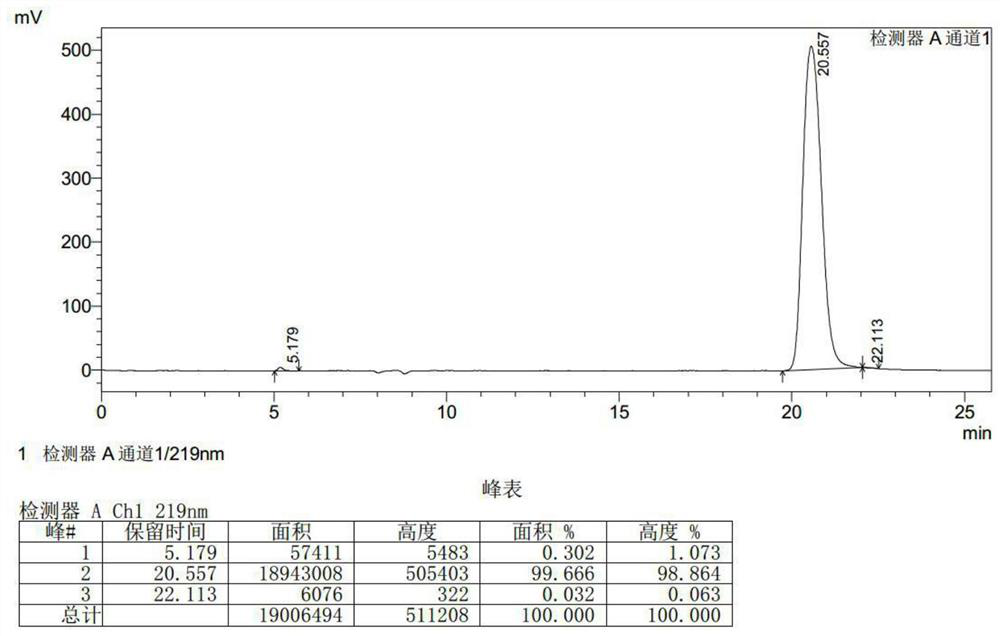
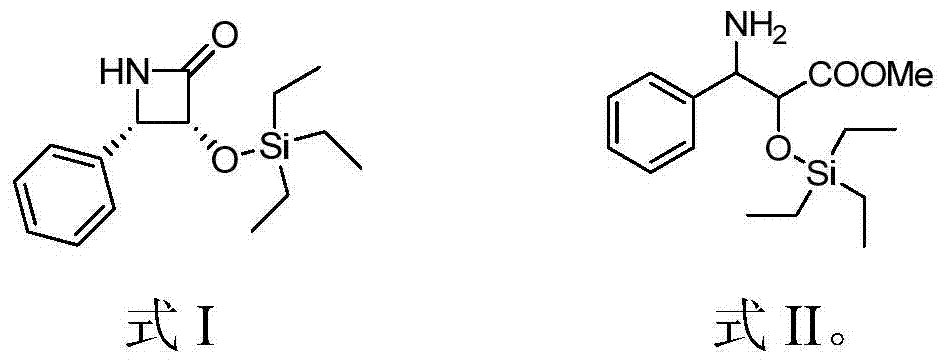
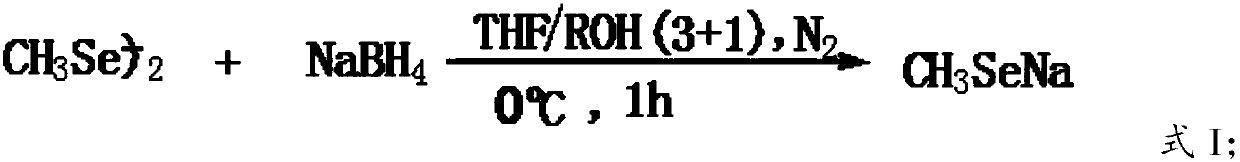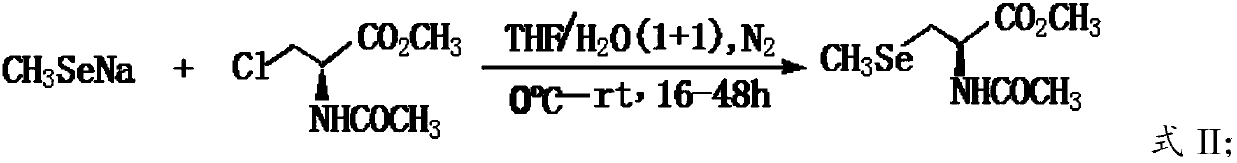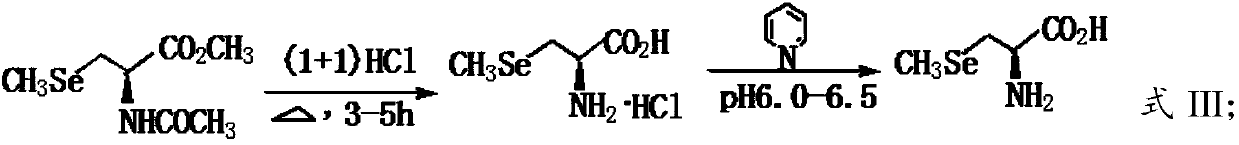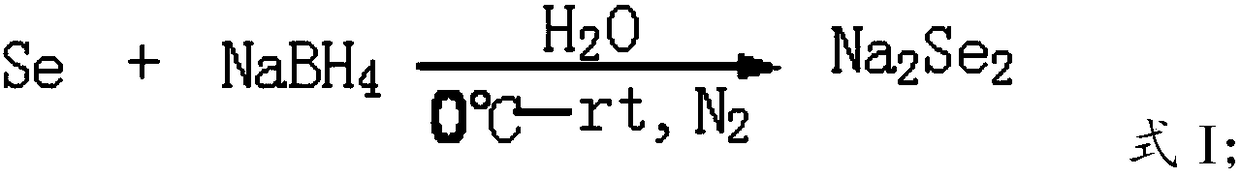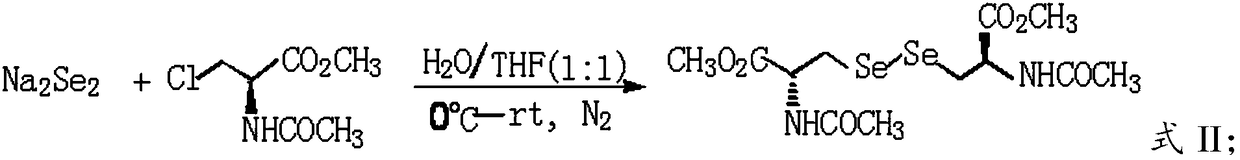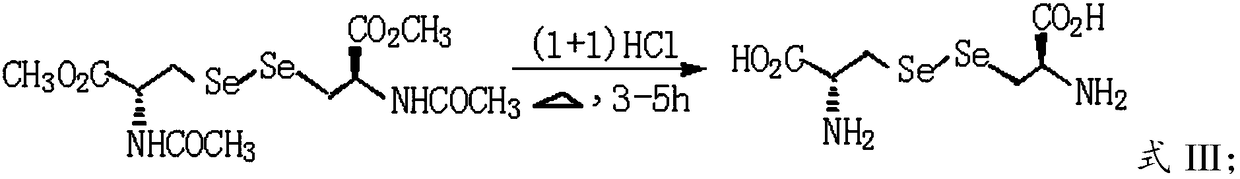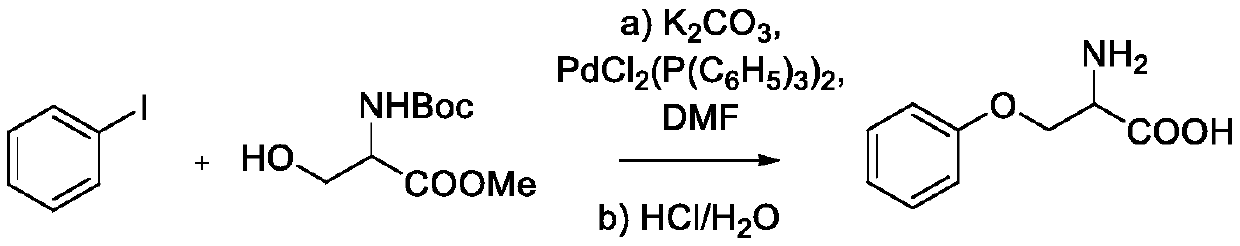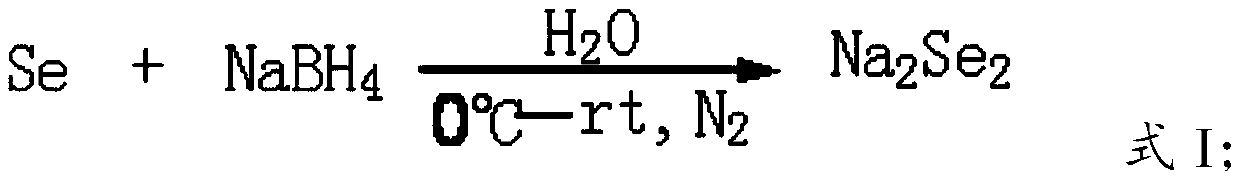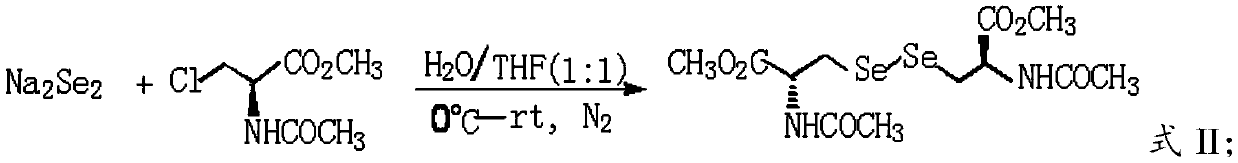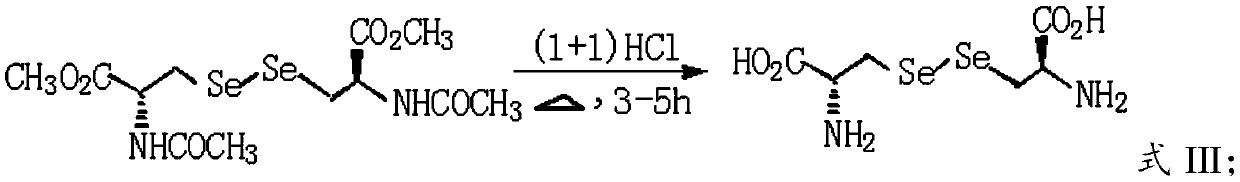Patents
Literature
41 results about "Serine methyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
D-Serine methyl ester hydrochloride 98% CAS Number 5874-57-7. Linear Formula HOCH 2 CH(NH 2)CO 2 CH 3 ·HCl . Molecular Weight 155.58 . MDL number MFCD00066121. eCl@ss 32160406 . PubChem Substance ID 24868202
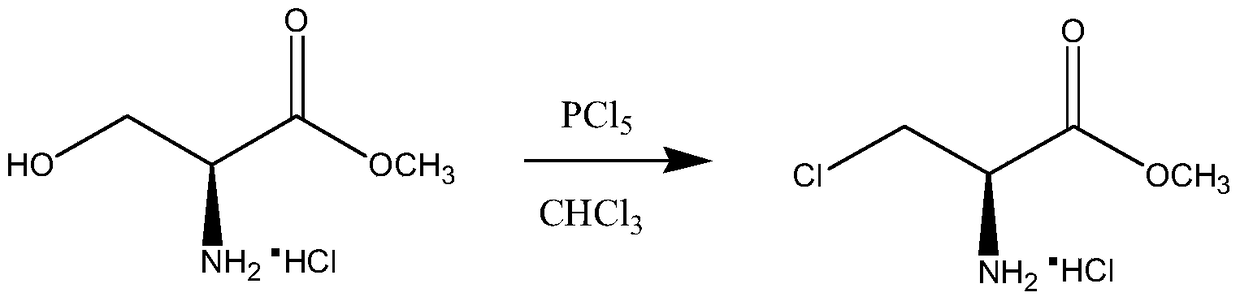
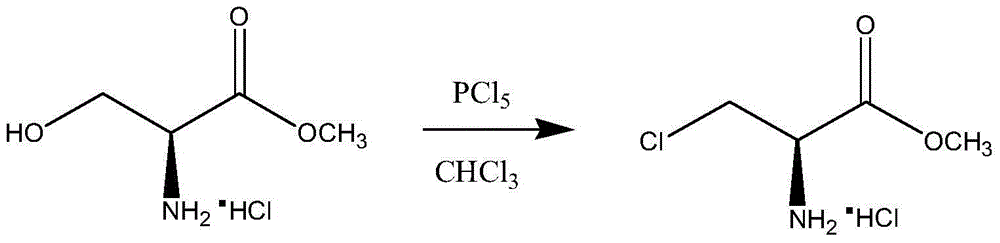
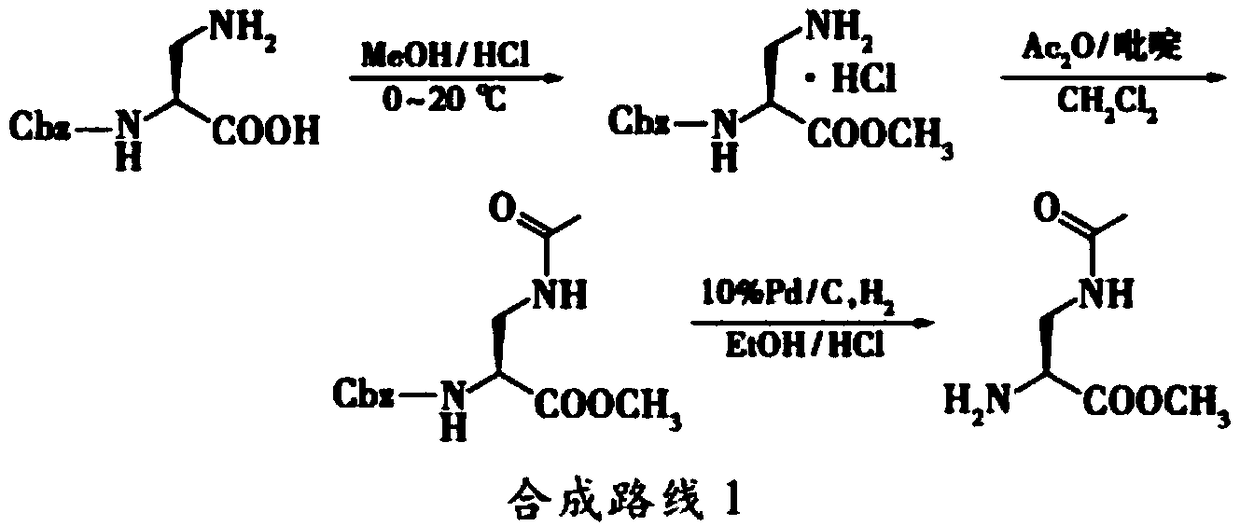
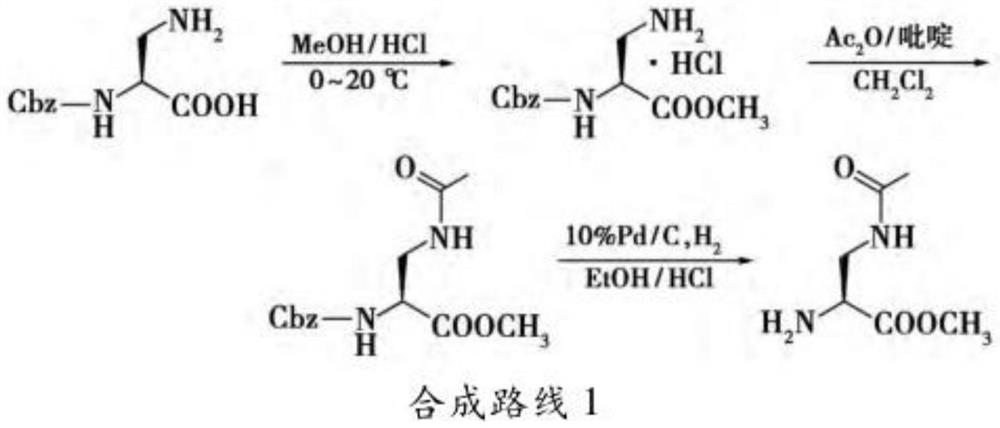
A synthetic method of (R)-methyl 2-amino-3-chloropropanoate hydrochloride
InactiveCN106518695AWide variety of sourcesSimple preparation processOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterFiltration
A synthetic method of (R)-methyl 2-amino-3-chloropropanoate hydrochloride is disclosed. The method includes steps of 1) dissolving D-serine into methanol, adding thionyl chloride dropwise into the methanol, performing a refluxing reaction to achieve methyl esterification, concentrating methanol until a product is dry, adding ethyl acetate, pulping at room temperature, and performing filtration, rinsing and drying to obtain D-serine methyl ester hydrochloride, and 2) adding the D-serine methyl ester hydrochloride and thionyl chloride into dichloroethane, performing chlorination for 24 h, performing filtration to obtain a crude (R)-methyl 2-amino-3-chloropropanoate hydrochloride product, decoloring the crude product in methanol, cooling, crystallizing and then performing filtration and drying. The method is high in yield, side products are less, a mother liquor after the reaction can be recycled after treatment, and the production cost is reduced. Tail gas generated by the method can be absorbed by a strong alkaline solution to form a salt, and the generated salt is then separated and reutilized, thus achieving environment friendly requirements.
Owner:安徽省诚联医药科技有限公司
Synthetic method for intermediate of benserazide hydrochloride
InactiveCN103951587AAvoid churnShorten the production cycleHydrazone preparationSerine methyl esterHydrazone
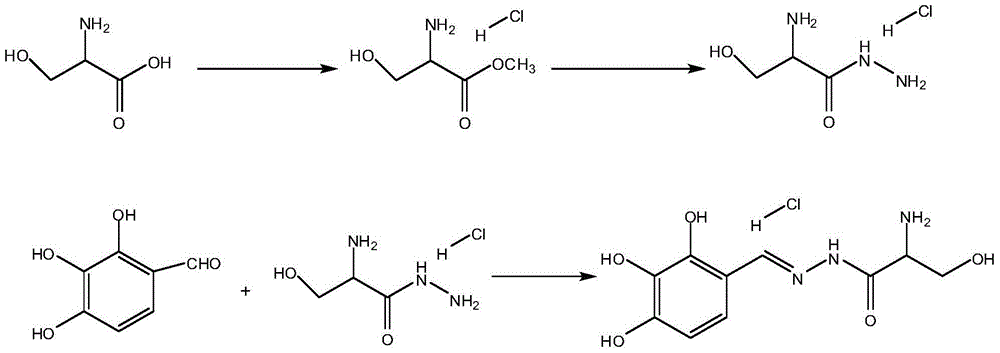
A provided synthetic method for an intermediate of benserazide hydrochloride comprises the following steps: step (a), taking DL-serine and thionyl chloride as initial raw materials, reacting to generate methyl-DL-serine hydrochloride, concentrating the reaction solution to dry, so as to obtain serine methyl ester, dissolving with methanol for usage in the reaction of step (b); step (b), adding serine methyl ester obtained in the step (a) into hydrazine hydrate, after reaction is finished, adding an alcohol, and adjusting ph to crystallize and further to obtain serine hydrazide; and step (c), reacting serine hydrazide obtained in the step (b) with 2,3,4-trihydroxybenzaldehyde in a methanol system, so as to obtain N-(DL-seryl)-2,3,4-trihydroxybenzaldehyde hydrazone. The preparation method disclosed by the invention is high in yield, low in cost, short in reaction period, environment-friendly and suitable for industrialized production.
Owner:ZHEJIANG NEXCHEM PHARMA
Preparation method of L-Se-methylselenocysteine
ActiveCN109535052AWide variety of sourcesCheap sourceOrganic chemistry methodsSelenocysteineSerine methyl ester
The invention provides a preparation method of L-Se-methylselenocysteine. The preparation method comprises the following steps: A) carrying out a nucleophilic substitution reaction on methyl hydroselenide sodium salt with N-acetyl-3-chloro-L-serine methyl ester to obtain N-acetyl-3-selenium methyl-L-selenocysteine methyl ester; and B) hydrolyzing the N-acetyl-3-selenium methyl-L-selenocysteine methyl ester obtained in the step A) in a hydrochloric acid solution to obtain the L-Se-methylselenocysteine. According to the invention, the N-acetyl-3-chloro-L-serine methyl ester is used as a chiral source reagent, and the obtained product is a single optically active substance, so that complex and tedious splitting process can be overcome. The process has the advantages that raw materials are easily available and cheap, operation is convenient, reaction conditions are mild, the product is single, separation is easy, the yield is high, and the method is suitable for industrial production.
Owner:河南希百康健康产业有限公司
Process for the preparation of lacosamide
InactiveCN106543018AOrganic compound preparationCarboxylic acid amides preparationSerine methyl esterNon-nucleophilic base
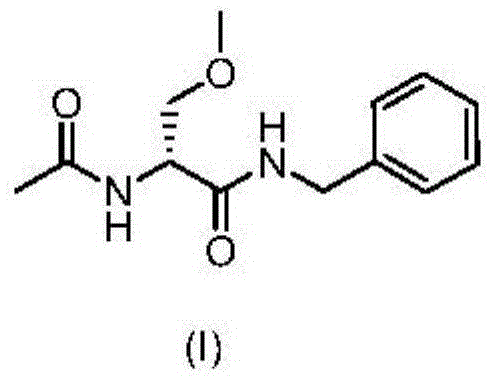
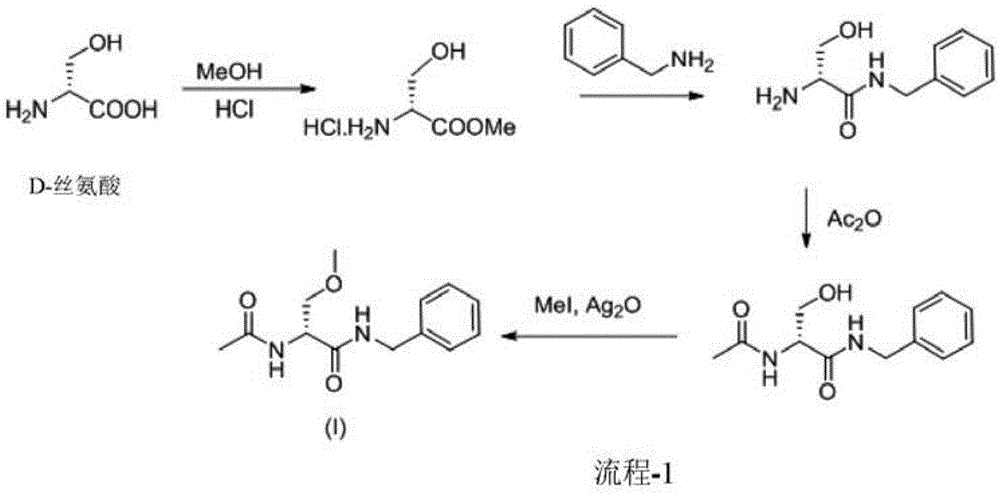
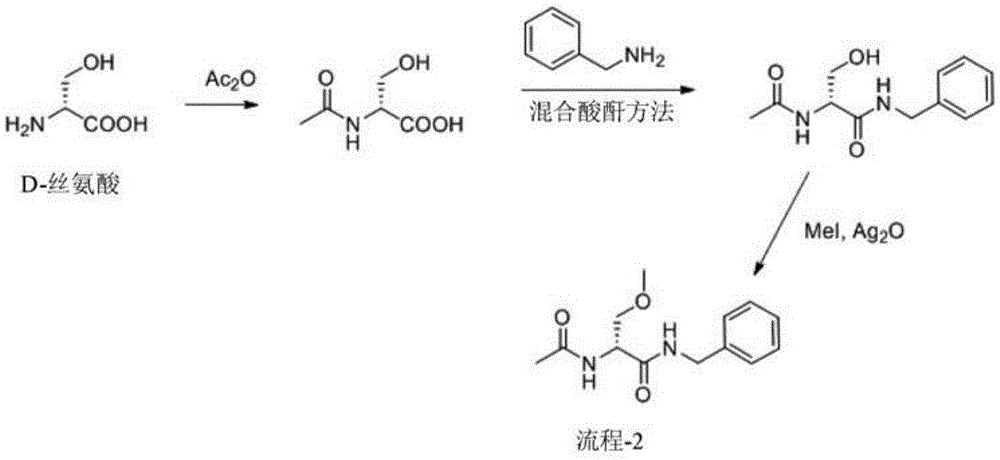
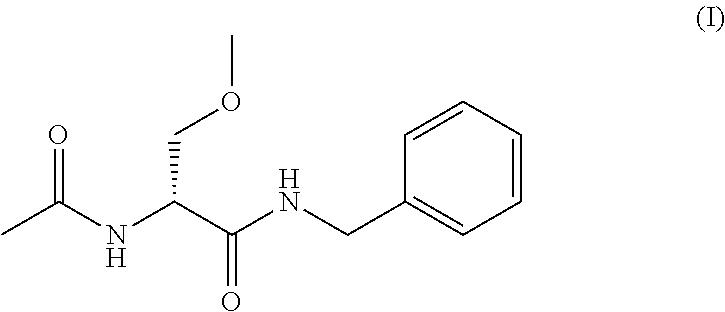
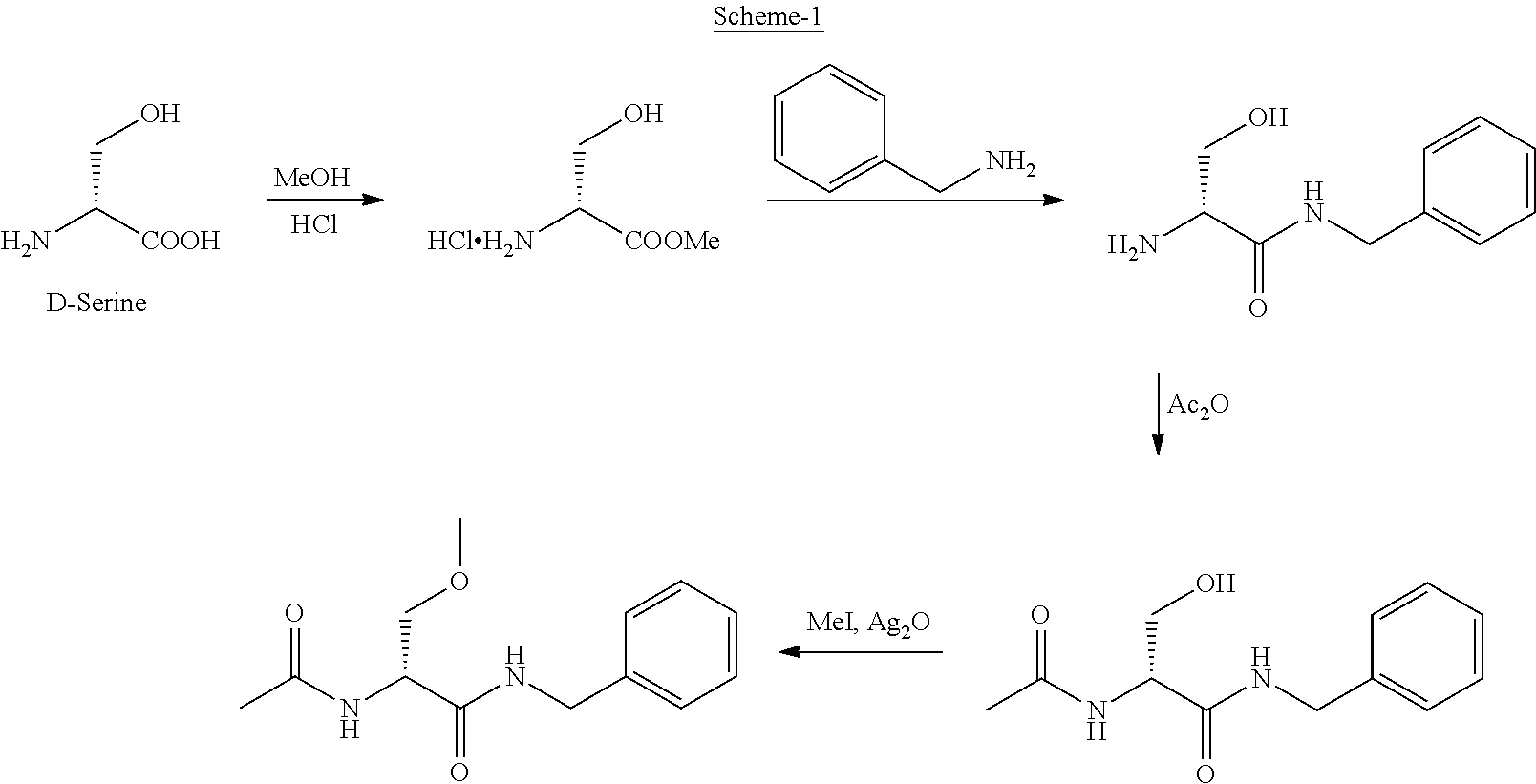
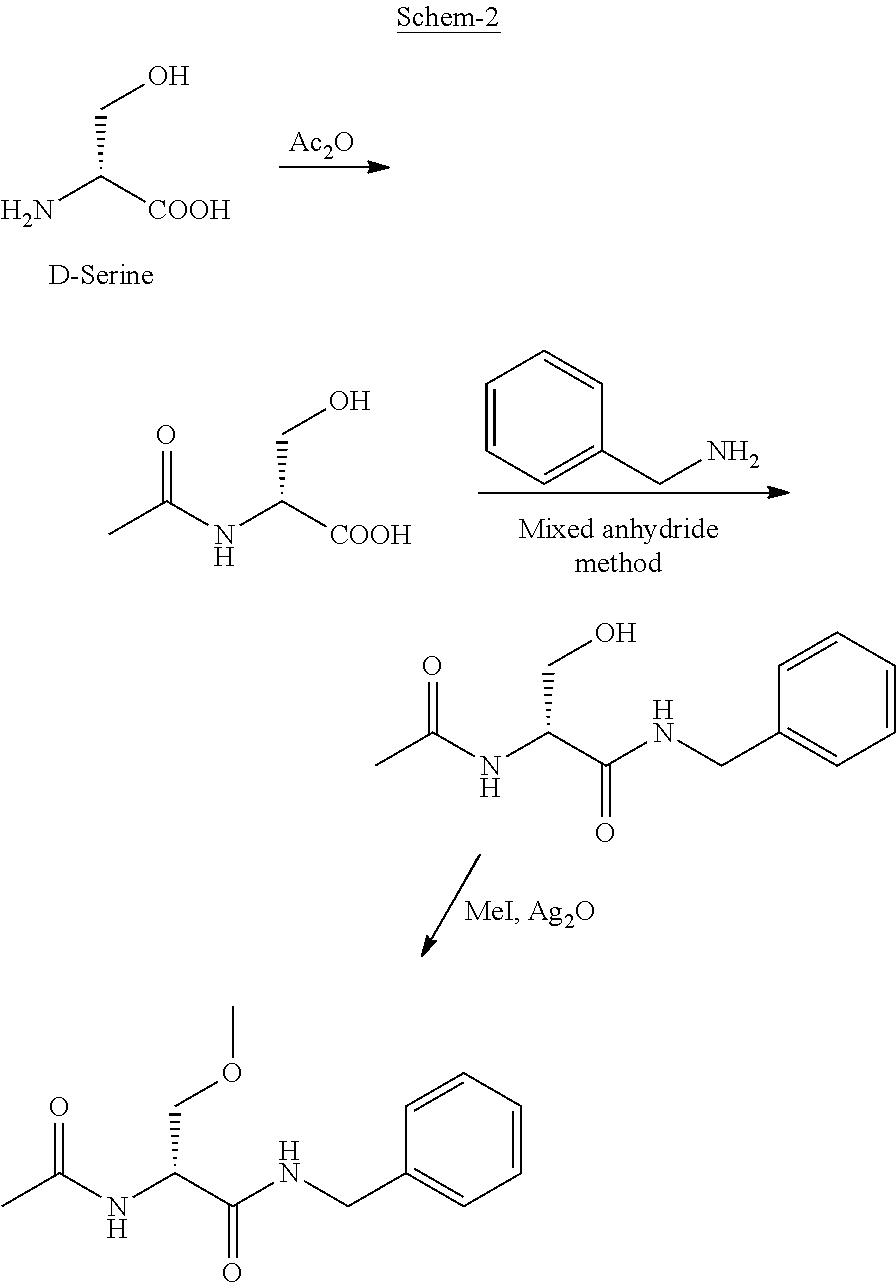
A novel process for the preparation of (R)-2-acetamido-N-benzyl-3-methoxypropionamide (Lacosamide) is described. It comprises reacting N-acetyl-D-serine methyl ester with benzylamine catalyzed by a non-nucleophilic base to obtain (R)-2-acetamido-2-N-benzyl-3-hydroxy propionamide followed by its methylation.
Owner:DIVI S LAB LTD
Method for preparing optically pure L-type selenium-methyl selenocysteine
ActiveCN108947881AHigh purityThe synthetic route is simpleOrganic chemistry methodsActivated carbonSerine methyl ester
The invention relates to a method for preparing an amino acid drug, and in particular to a method for preparing optically pure L-type selenium-methyl selenocysteine. The method comprises the steps ofmixing N-acetyl-3-chloro-L-serine methyl ester with methylselenol salt to obtain N-acetyl-3-methyl selenyl-L-serine methyl ester; mixing with a hydrochloric acid solution and carrying out hydrolysis reaction to obtain selenium-methyl selenocysteine; dissolving the selenium-methyl selenocysteine in water, adding a solvent A and uniformly mixing; adding activated carbon to decolorize, filtering andcollecting filtrate; and adding a solvent B into filtrate, uniformly mixing, cooling, standing for more than 1 hour and collecting crystals to obtain the optically pure L-type selenium-methyl selenocysteine. The method disclosed by the invention has the characteristics of being simple in process, high in product optical purity, green and friendly to environment and is suitable for large-scale industrial production.
Owner:济源希健生物医药科技发展有限公司
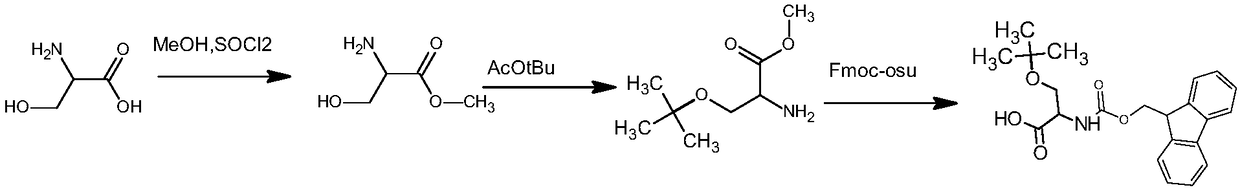
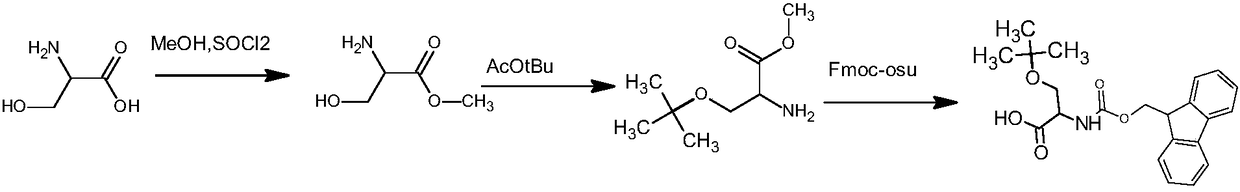
Preparation method of fmoc-O-tert-butyl-L-serine
InactiveCN109265370ALow toxicityLow priceCarbamic acid derivatives preparationOrganic compound preparationSerine methyl esterTert butyl
The invention discloses a preparation method of fmoc-O-tert-butyl-L-serine. The method comprises the steps that 1, L-serine and a methanol solution are added into a reaction container, and under stirring, SOCl2 is added dropwise for reflux reaction to obtain L-serine methyl ester hydrochloride; 2, the L-serine methyl ester hydrochloride is added into tert-butyl acetate, and a catalyst is added forreaction to obtain O-tert-butyl-L-serine methyl ester; 3, the O-tert-butyl-L-serine methyl ester is added into alkaline liquid for saponification reaction to obtain an O-tert-butyl-L-serine aqueous solution; 4, an organic solvent and NaCO3 are added into O-tert-butyl-L-serine, then fmoc n-hydroxysuccinimide este is added to adjust a pH value to be 8-10, and extraction separation is conducted to obtain the fmoc-O-tert-butyl-L-serine. In the preparation method, the L-serine, methanol and SOCl2 are adopted as raw materials, and through the reflux reaction, the L-serine methyl ester hydrochlorideis prepared; the reaction is carried out in liquid phases respectively, and is safe and pollution-free.
Owner:四川什邡市三高生化实业有限公司
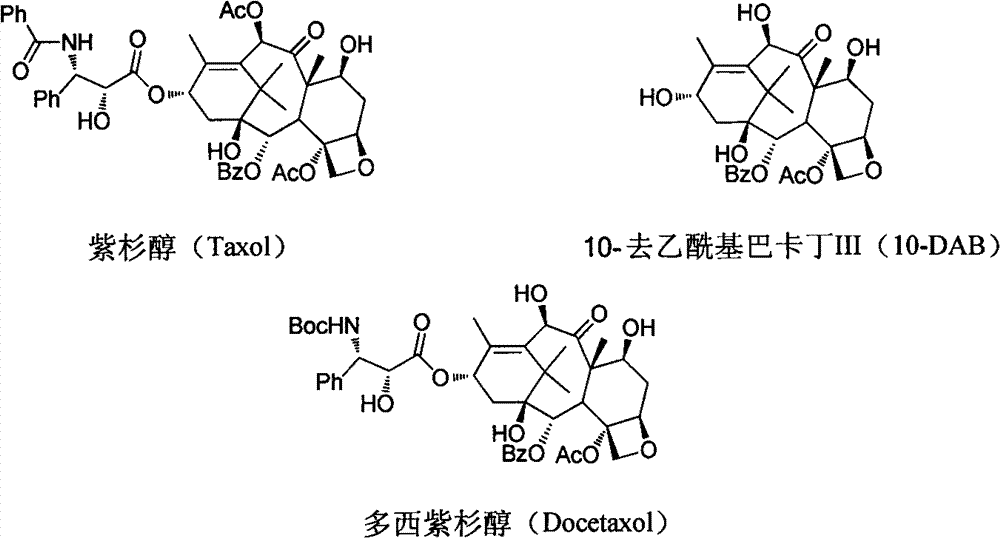
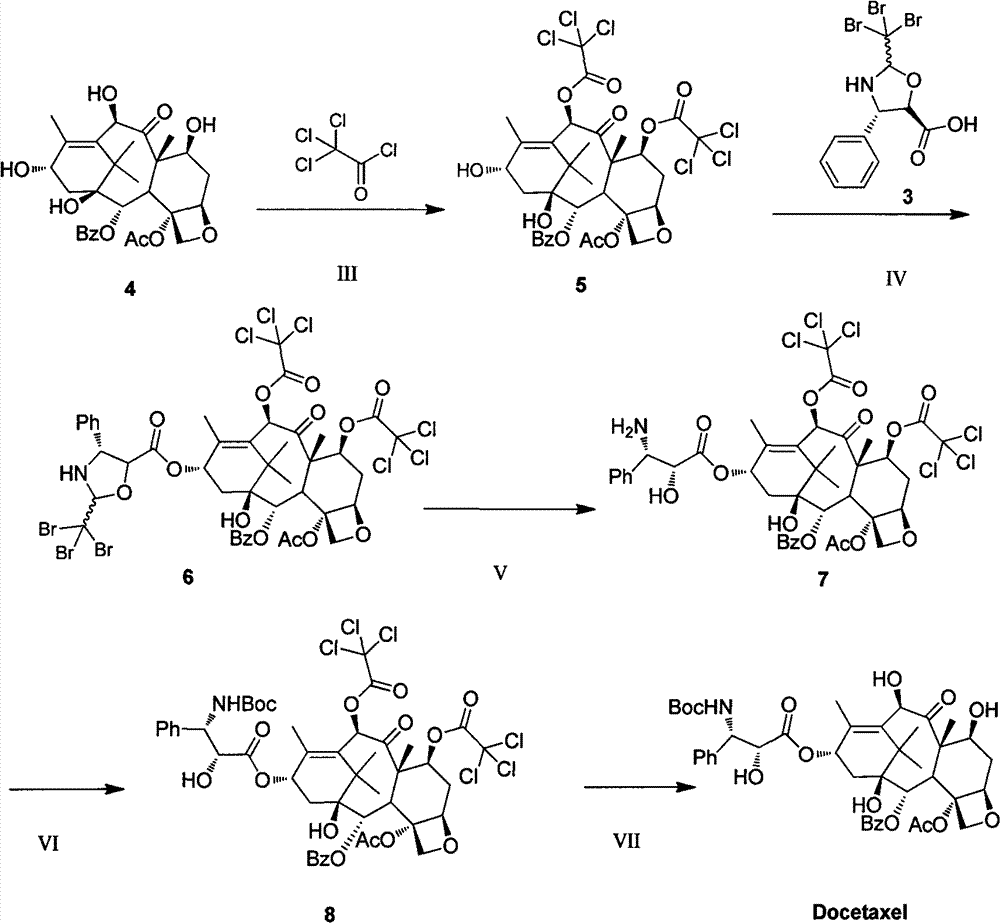
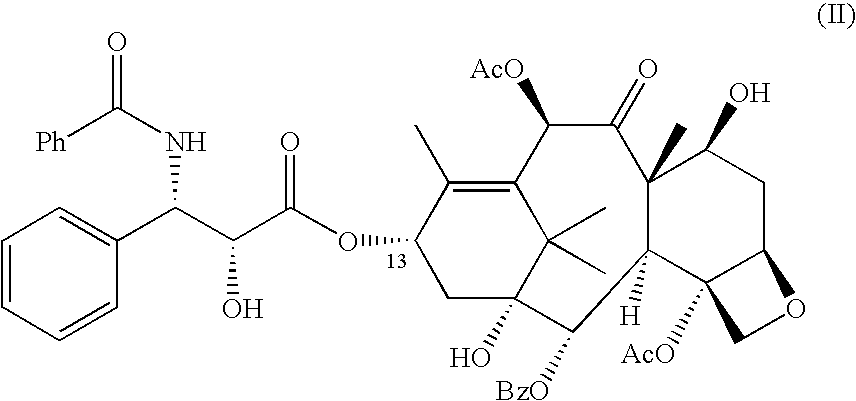
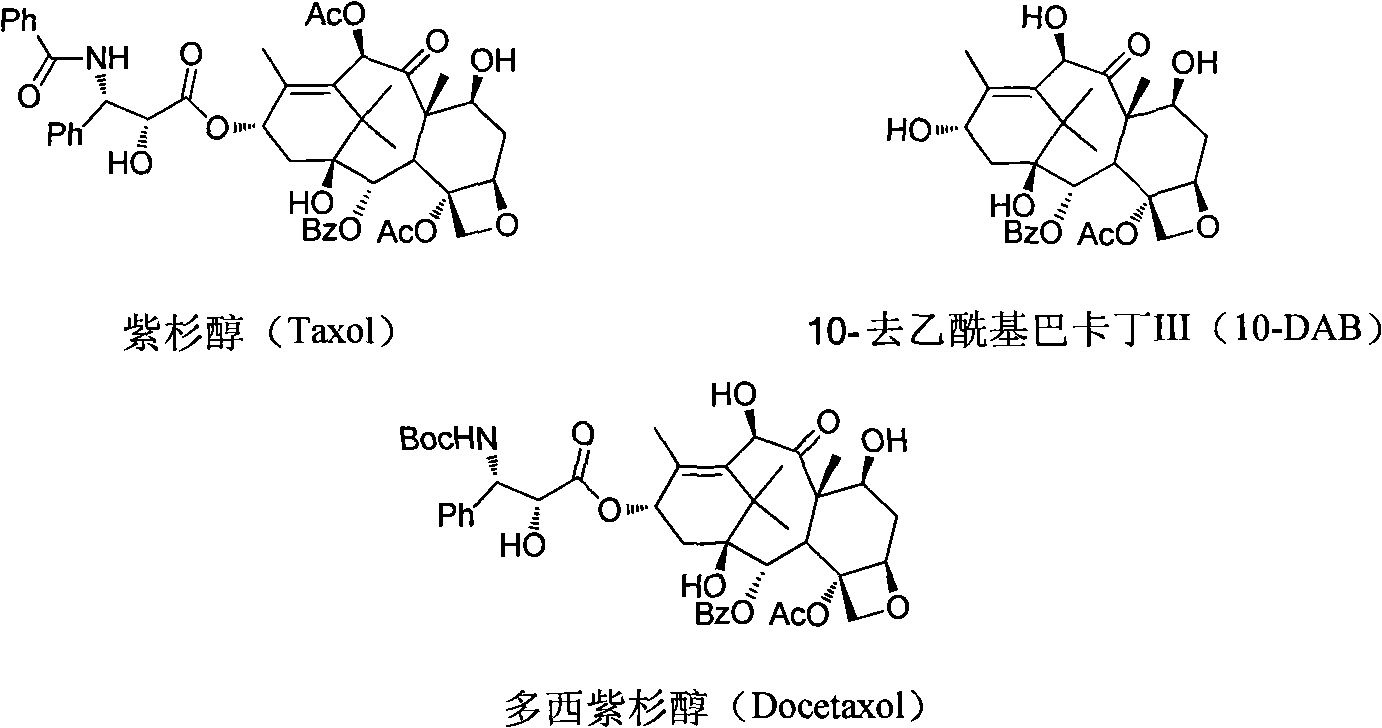
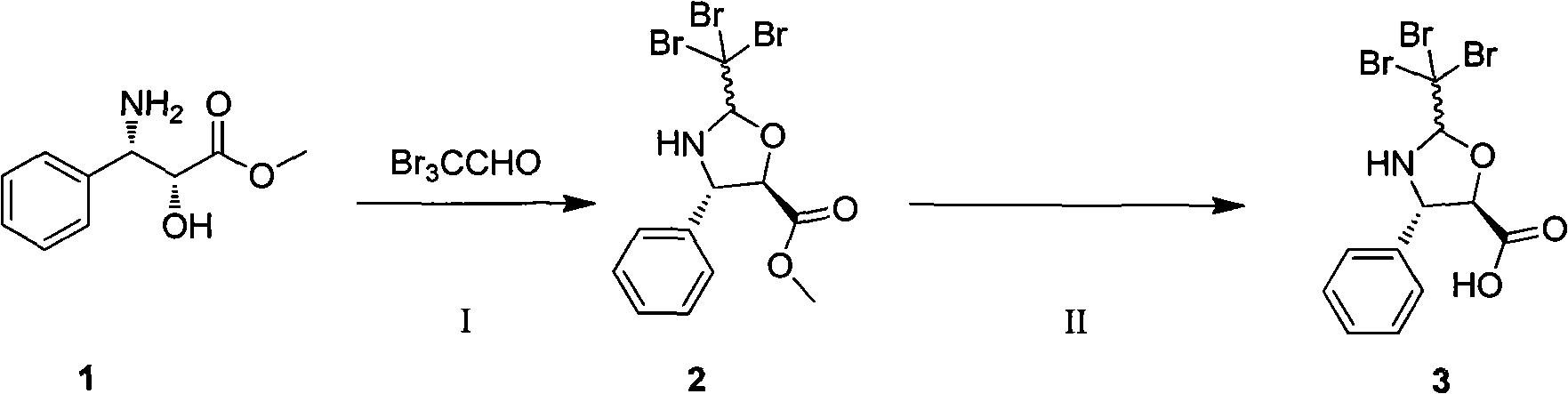
Improved method for semi-synthesizing docetaxel
InactiveCN102887876AOvercome the disadvantage of many reaction stepsSolve the problem of isomeric impuritiesOrganic chemistryBulk chemical productionSerine methyl esterDocetaxel-PNP
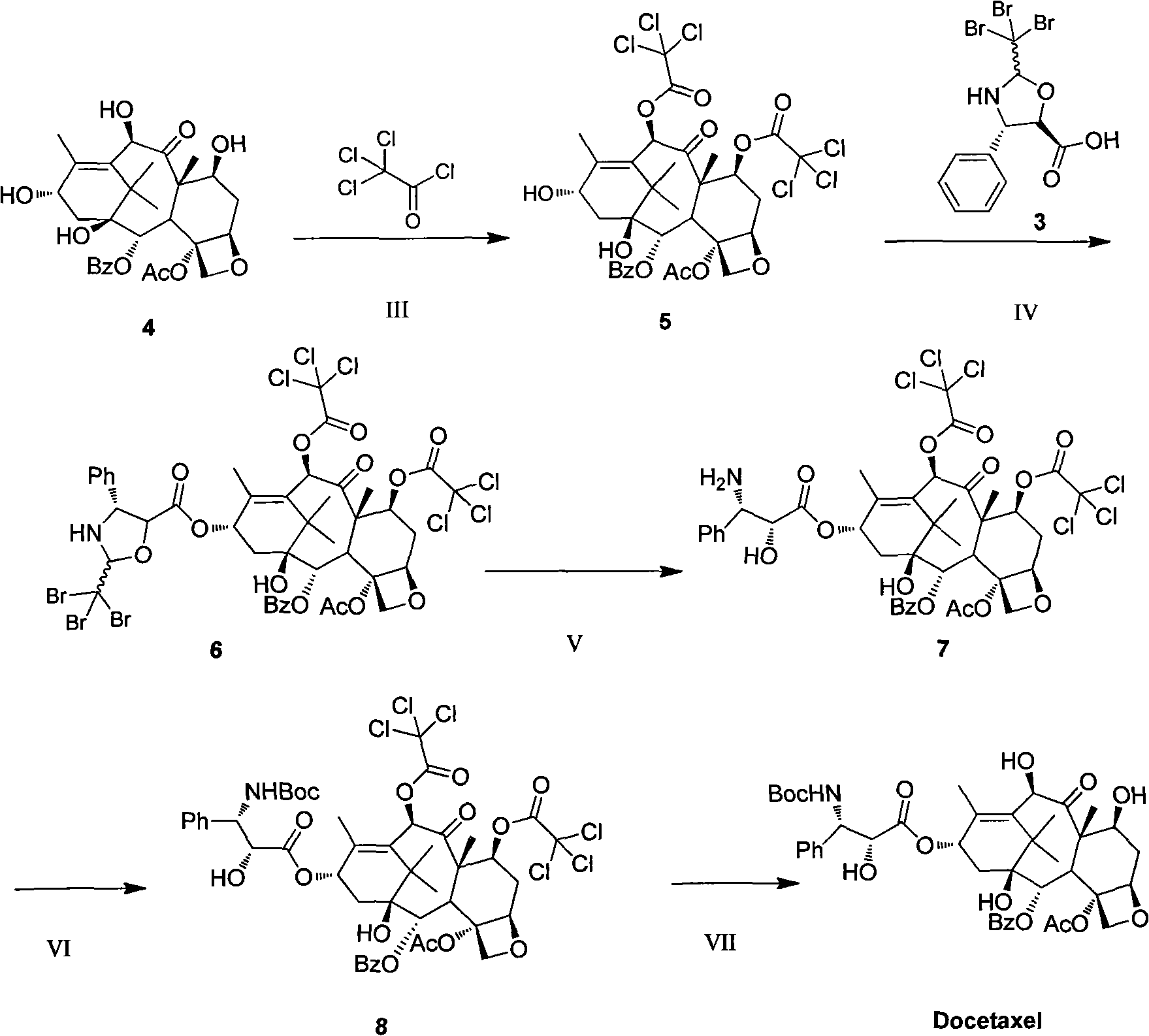
The invention discloses an improved method for semi-synthesizing docetaxel. The method comprises the following steps of: protecting hydroxyl radicals at the 7th position and the 10th position of 10-deacetyl baccatin III which is taken as an initial raw material, introducing an optical rotation side chain at the 13th position of the 10-deacetyl baccatin III to synthesize an intermediate, and finally, hydrolyzing to obtain a target product, namely the docetaxel. The side chain is synthesized by reacting (2R,3S)-3-phenylpropionate with tribromoacetaldehyde to obtain an oxazoline intermediate, and hydrolyzing the oxazoline intermediate. The preparation method is simple, few side reactions are performed, the yield and the purity are high, isomer byproducts are avoided, the content of impurities is low, and the cost is greatly reduced, so the commercial preparation of the docetaxel can be promoted. The method has an important significance and a huge economic benefit for protecting the species and resource of Taxus chinensis plants, reducing the treatment cost of tumor and the like.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
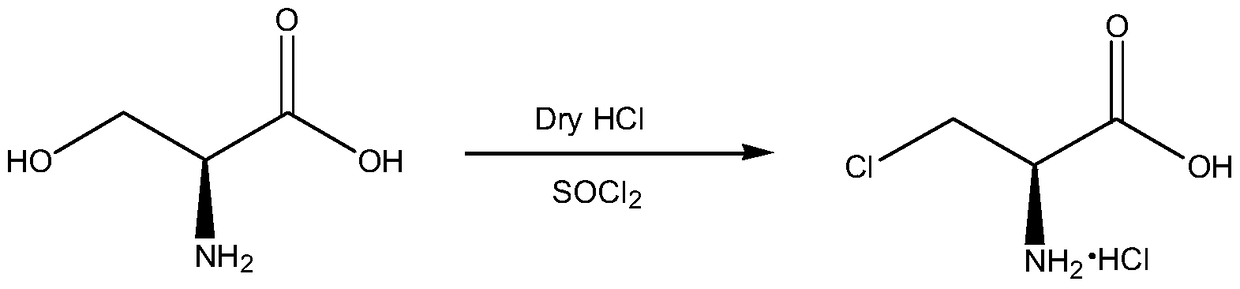
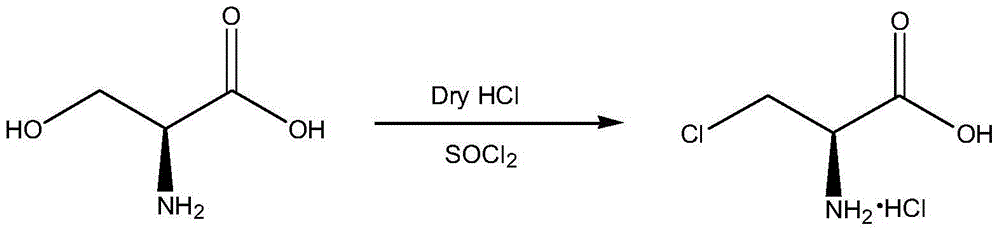
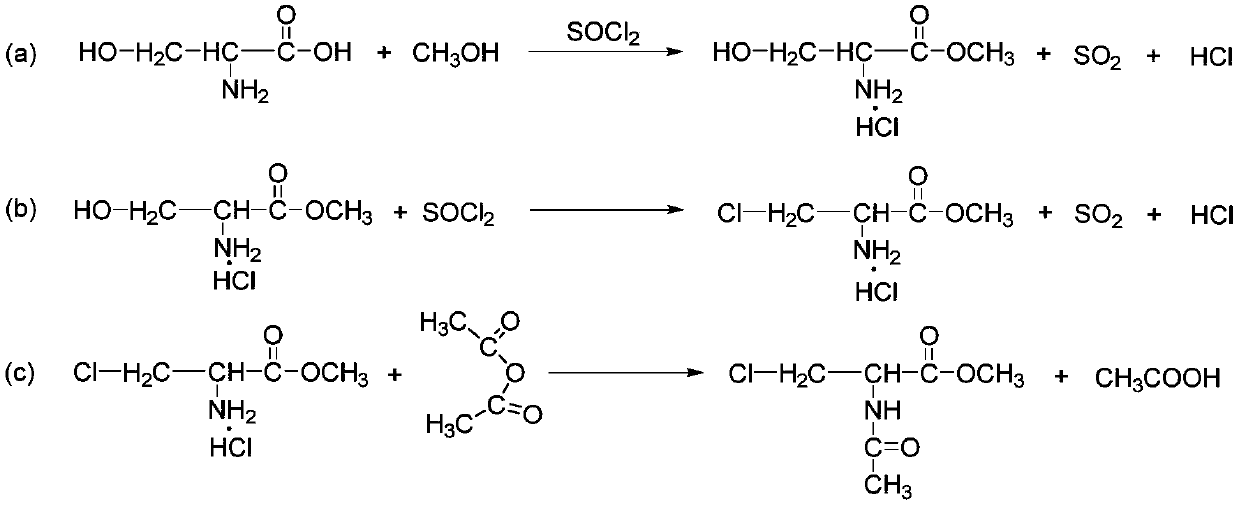
Synthetic method of 3-chloro-L-alanine methyl ester hydrochloride
InactiveCN110590587AHigh puritySimple processOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterTemperature control mode
The invention discloses a synthetic method of 3-chloro-L-alanine methyl ester hydrochloride. The method comprises the following steps: adding L-serine into a first solvent, performing cooling to 5-10DEG C, adding thionyl chloride A dropwise, performing heating to 38 DEG C after adding is completed, performing a reaction for 48 h, performing cooling crystallization on the mixture, performing centrifugation and performing drying to obtain L-serine methyl ester hydrochloride; and adding the L-serine methyl ester hydrochloride into a second solvent, adding thionyl chloride B dropwise, performinga sectional temperature control reaction, performing cooling to 15-25 DEG C after the reaction is finished, adding water for layering, and treating the obtained water phase to obtain the 3-chloro-L-alanine methyl ester hydrochloride. By adopting the sectional temperature control mode, the method has the advantages of short reaction time, mild reaction conditions, a high yield and fewer byproducts,and is convenient for enhancing the yield and product purity; the L-serine methyl ester hydrochloride does not need to be purified, so that the process is optimized; and water is added for layering,an organic phase is extracted for multiple times, adsorption is performed by activated carbon in a water phase, so that the product yield and the product purity are further improved.
Owner:湖北宇阳药业有限公司
Preparation method of L-selenocystine
ActiveCN109369482AWide variety of sourcesMild reaction conditionsOrganic chemistry methodsRotatory powerSerine methyl ester
The invention provides a preparation method of L-selenocystine. The preparation method comprises the following steps: A) carrying out nucleophilic substitution reaction on sodium diselenide and N-acetyl-3-chloro-L-serine methyl ester to obtain N,N'-diacetyl-L-selenocystine dimethyl ester; B) hydrolyzing the N,N'-diacetyl-L-selenocystine dimethyl ester in a hydrochloric acid solution to obtain theL-selenocystine. The preparation method takes an N-acetyl-3-chloro-L-serine methyl ester chiral raw material as a substrate and sodium borohydride as a reducingagent, has the characteristics of easy-to-obtain and cheap raw materials, convenience for operation, moderate reaction conditions, single product, easiness for separation and high yield and the like and is suitable for industrial production. A test result proves that the specific rotatory power of the preparation method provided by the invention can reach that [alpha]D<20> is equal to -29.5 DEG (C 0.5, 0.1 MNaOH) and the highest yield of a product is 80.6 percent.
Owner:河南希百康健康产业有限公司
Preparation method of chiral 1-tert-butyl-3-methyl-6-methylpiperazine-1, 3-diformate
PendingCN112321515AReduce manufacturing costRaw materials are easy to getOrganic chemistrySerine methyl esterCombinatorial chemistry
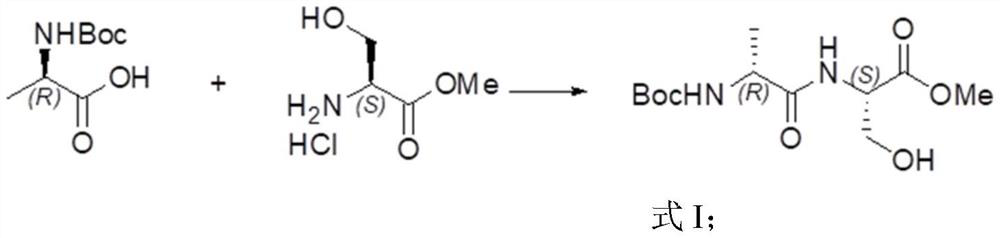
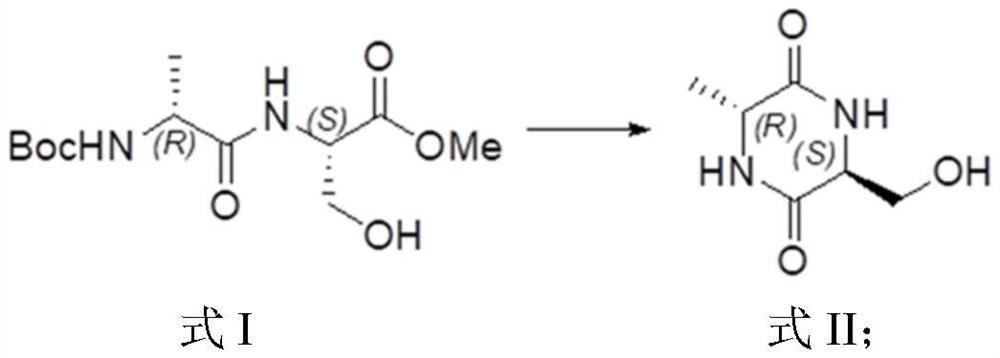
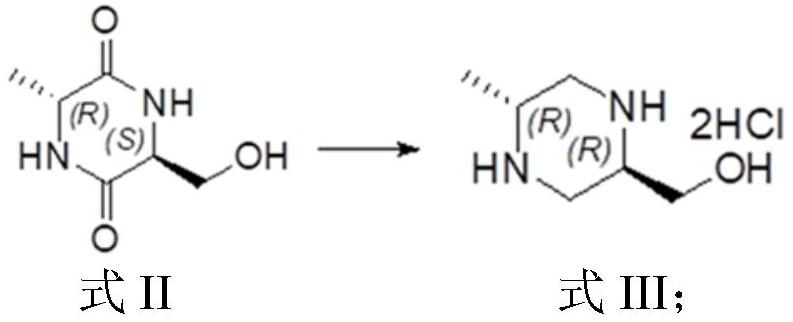
The invention provides a preparation method of chiral 1-tert-butyl-3-methyl-6-methylpiperazine-1, 3-diformate, and particularly relates to a preparation method of (3R,6R) -1-tert-butyl-3-methyl-6-methylpiperazine-1, 3-diformate. The preparation method comprises the steps of taking Boc-D-alanine and L-serine methyl ester hydrochloride as initial raw materials, and preparing the chiral 1-tert-butyl-3-methyl-6-methylpiperazine-1, 3-diformate through condensation reaction, deamination protection, ring closing reaction, reduction reaction, amino protection, oxidation reaction, ring closing reactionand ring opening reaction. According to the preparation method provided by the invention, the Boc amino protective agent is used for protecting amino, so that the production cost is reduced, the rawmaterials are easy to obtain, the conditions are mild, the yield is relatively high, and the preparation method can be applied to large-scale industrial production.
Owner:SHANGHAI BALMXY PHARMA CO LTD
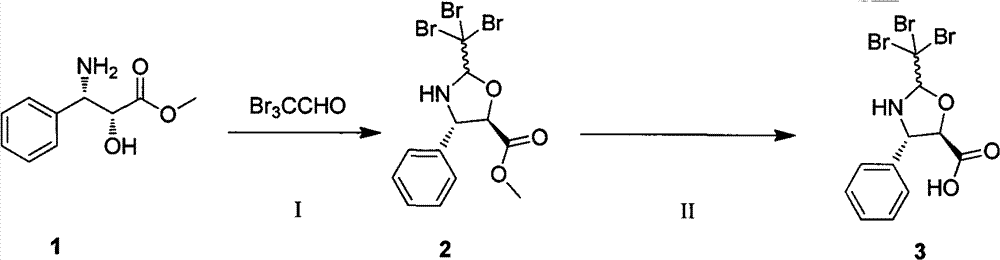
Synthetic method of paclitaxel side chain
The invention discloses a synthetic method of a paclitaxel side chain. The method comprises the steps of taking (2R, 3S)-3-phenyl isoserine hydrochloride as a raw material; carrying out esterificationreaction under the participation of methanol and thionyl chloride to obtain (2R, 3S)-phenyl isoserine methyl ester; then preparing (2R, 3S)-N-benzoyl-phenyl isoserine methyl ester through a benzoylation reaction; preparing (4S, 5R)-5-methoxycarbonyl-2-(4-methoxyphenyl)-4-phenyl-3-benzoyl-1,3-oxazolidine through a cyclization protection reaction; finally, obtaining a paclitaxel side chain crude product through hydrolysis, and further purifying the paclitaxel side chain crude product through recrystallization to obtain a paclitaxel side chain finished product. The method is simple and easy to operate, short in production period, low in cost, high in purification efficiency and suitable for industrial application and market popularization.
Owner:YUNNAN HANDE BIO TECH
Preparation method of 1-aryl-2-amidogen-1,3-propylene glycol hydrochloride derivative
InactiveCN108017549AWide variety of sourcesEasy to getOrganic compound preparationAmino-hyroxy compound preparationSerine methyl ester1,3-Propanediol
The invention discloses a preparation method of a 1-aryl-2-amidogen-1,3-propylene glycol hydrochloride derivative. The method comprises the steps that N-Boc-D-serine methyl ester is used as an initialraw material and prepared through amidogen fixation, reduction reaction, Grignard reaction, Grignard butt joint, acetonylidene removal and salinization reaction. The preparation method has the advantages that the source of the initial raw material N-Boc-D-serine methyl ester is wide, easy to obtain and moderate in cost, the synthesis process is simple in route, and the preparation cost of products is lowered; acetonylidene is applied to protection of amidogen and aliphatic hydroxyl groups, the side reaction caused by active amidogen and aliphatic hydroxyl groups is effectively avoided, impurities are reduced, and the product purity and reaction yield are increased; the target products exist in a hydrochloride compound mode, the stability of the products is improved, the products are easily stored and conveyed, and the preparation method is suitable for large-scale industrialized production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Green and efficient method for preparing L-selenium methylselenocysteine
ActiveCN110628839AAvoid instabilityReduced purificationOrganic chemistryChemical recyclingEnvironmental resistanceMethylselenocysteine
The present invention discloses a green and efficient method for preparing L-selenium methylselenocysteine. Firstly, L-serine methyl ester hydrochloride is used as a starting raw material and an acetylation, chlorination and methylselenization one-pot method is used to synthesize a reaction substrate, and then the obtained reaction substrate is hydrolyzed under an action of enzyme catalysis to generate L-selenomethylselenocysteine. The one-pot method is used to prepare the reaction substrate, overcomes instability of intermediates and also reduces tedious steps of separation and purification of the intermediates; the enzyme catalysis hydrolysis is used to avoid environmental pollution and equipment corrosion caused by strong acid hydrolysis and overcome disadvantages of selenium-ether bondrupture, product racemization, etc. Characteristics are as follows: the raw materials are easy to obtain and cheap in price, the method is convenient to operate, reaction conditions are mild, green and environmentally protectively, and the product is simple, easy for separation, high in yield and suitable for industrial production.
Owner:河南希百康健康产业有限公司
A method for preparing optically pure L-type selenium-methylselenocysteine
ActiveCN108947881BThe synthetic route is simpleRaw materials are cheap and easy to getOrganic chemistry methodsMethylselenocysteineActivated carbon
The invention relates to a method for preparing an amino acid drug, and in particular to a method for preparing optically pure L-type selenium-methyl selenocysteine. The method comprises the steps ofmixing N-acetyl-3-chloro-L-serine methyl ester with methylselenol salt to obtain N-acetyl-3-methyl selenyl-L-serine methyl ester; mixing with a hydrochloric acid solution and carrying out hydrolysis reaction to obtain selenium-methyl selenocysteine; dissolving the selenium-methyl selenocysteine in water, adding a solvent A and uniformly mixing; adding activated carbon to decolorize, filtering andcollecting filtrate; and adding a solvent B into filtrate, uniformly mixing, cooling, standing for more than 1 hour and collecting crystals to obtain the optically pure L-type selenium-methyl selenocysteine. The method disclosed by the invention has the characteristics of being simple in process, high in product optical purity, green and friendly to environment and is suitable for large-scale industrial production.
Owner:济源希健生物医药科技发展有限公司
A kind of synthetic method of acrylic acid derivative
Owner:成都道合尔医药技术有限公司
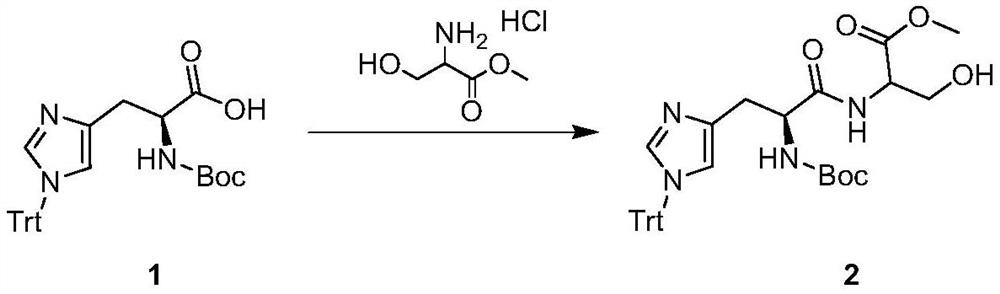
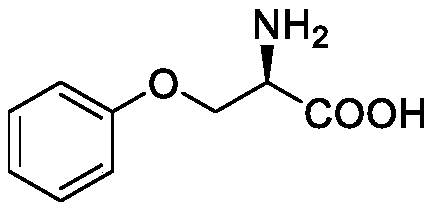
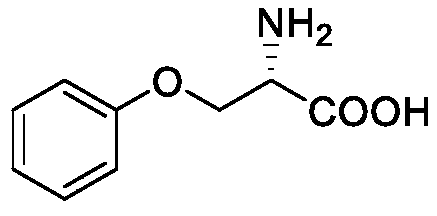
Synthesis method of multi-configuration O-phenyl-serine compound
ActiveCN111333528AHigh yieldEasy to optimizeCarbamic acid derivatives preparationOrganic compound preparationSerine methyl esterPtru catalyst
The invention relates to a synthesis method of a multi-configuration O-phenyl-serine compound. The method comprises the following steps: (1) in the presence of a solvent, alkali and a palladium catalyst, reacting a compound shown as formula I and a compound shown as formula II under the protection of inert gas to obtain an intermediate compound; (2) carrying out deprotection reaction on the intermediate compound to prepare an O-phenyl-serine compound shown as formula III; wherein the structural formula of the compound shown as formula I is shown in the specification, the structural formula ofthe compound shown as formula II is shown in the specification, and the structural formula of the compound shown as formula III is shown in the specification, wherein in the formula I and formula III,R is one of H, alkyl, alkoxy and halogen. According to the synthesis method disclosed by the invention, the palladium catalyst is selected as the catalyst, so that the reaction of mono-substituted iodobenzene and multi-configuration N-tert-butyloxycarbonyl-serine methyl ester can be effectively catalyzed, the reaction is promoted, the reaction is simple and easy to operate, mild, low in toxicity,and high in product yield.
Owner:苏州爱玛特生物科技有限公司
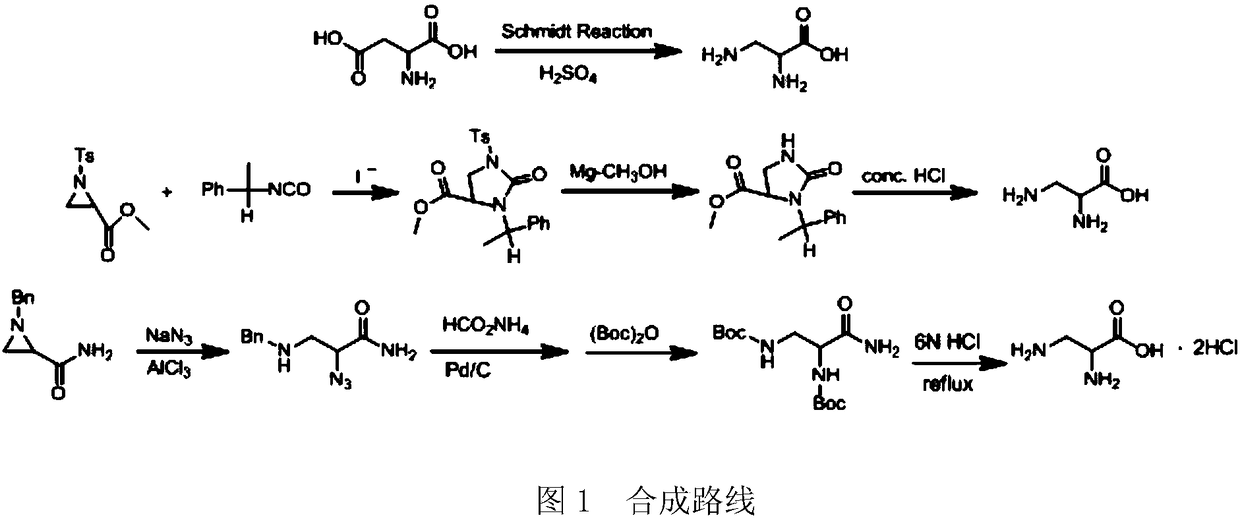
A kind of synthetic method of d-cycloserine intermediate
ActiveCN106146327BImprove operational safetyHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterOrganic solvent
Disclosed is a method of synthesizing a 3-halo-D-alanine methyl ester or an acid salt thereof. By reacting a D-serine methyl ester or an acid salt thereof and a halogenation reagent in an organic solvent, the method prepares a 3-halo-D-alanine methyl ester or an acid salt thereof. The compound is an important intermediate for preparing a D-cycloserine. The method and process employed in the present invention are easy to operate and highly safe, require a moderate reaction condition, have a high yield, and are low in cost, and reduce waste acid generation considerably and are suitable for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Synthetic method of D-cycloserine intermediate
ActiveCN106146327AImprove operational safetyHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterOrganic solvent
The invention relates to a synthetic method of a D-cycloserine intermediate. According to the method, 3-halo-D-alanine methyl ester or an acid salt of 3-halo-D-alanine methyl ester is prepared from D-serine methyl ester or an acid salt of D-serine methyl ester and a halogenating agent through reaction in an organic solvent, and is an important intermediate for preparing D-cycloserine. The synthetic method adopts a process simple and convenient to operate, is high in safety, mild in reaction condition, high in yield and low in cost, greatly reduces production of waste acid and is quite suitable for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A kind of synthetic method of d-cycloserine
Owner:NANJING REDWOOD FINE CHEM CO LTD
A kind of preparation method of L-selenocystine
ActiveCN109369482BWide variety of sourcesMild reaction conditionsOrganic chemistry methodsSerine methyl esterSodium selenide
The invention provides a preparation method of L-selenocystine. The preparation method comprises the following steps: A) carrying out nucleophilic substitution reaction on sodium diselenide and N-acetyl-3-chloro-L-serine methyl ester to obtain N,N'-diacetyl-L-selenocystine dimethyl ester; B) hydrolyzing the N,N'-diacetyl-L-selenocystine dimethyl ester in a hydrochloric acid solution to obtain theL-selenocystine. The preparation method takes an N-acetyl-3-chloro-L-serine methyl ester chiral raw material as a substrate and sodium borohydride as a reducingagent, has the characteristics of easy-to-obtain and cheap raw materials, convenience for operation, moderate reaction conditions, single product, easiness for separation and high yield and the like and is suitable for industrial production. A test result proves that the specific rotatory power of the preparation method provided by the invention can reach that [alpha]D<20> is equal to -29.5 DEG (C 0.5, 0.1 MNaOH) and the highest yield of a product is 80.6 percent.
Owner:河南希百康健康产业有限公司
Process for the preparation of lacosamide
ActiveUS9447024B1Organic compound preparationCarboxylic acid amides preparationSerine methyl esterNon-nucleophilic base
A novel process for the preparation of (R)-2-acetamido-N-benzyl-3-methoxypropionamide (Lacosamide) is described. It comprises reacting N-acetyl-D-serine methyl ester with benzylamine catalyzed by a non-nucleophilic base to obtain (R)-2-acetamido-2-N-benzyl-3-hydroxy propionamide followed by its methylation.
Owner:DIVI S LAB LTD
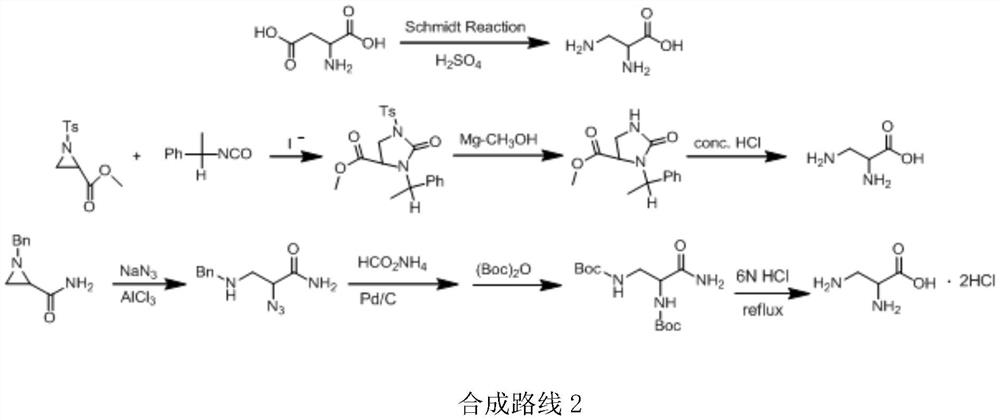
Preparation method of 2,3-diamido methyl propionate
ActiveCN109251150AStarting materials are readily availableMild reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterHydrazine compound
The invention provides a preparation method of 2,3-diamido methyl propionate. According to the method, serine is used as a raw material, in methyl alcohol, thionyl chloride is used as a catalyst for preparing serine methylester, then the serine methylester is reacted with triphenyl chloromethane to generate an intermittent product 1, a Trt group is introduced, anhydrous tetrahydrofuran is used asa solvent of the intermittent product 1, under catalysis of triphenylphosphine and DIAD, the intermittent product 1 is reacted with phthalimide to generate an intermittent product 2, a Pht group is introduced, under hydrazine hydrate action, the Pht group is removed from the intermittent product 2 to generate an intermittent product 3, and finally, in hydrochloric acid alcohol, the Trt group is removed from the intermittent product 3 to obtain the final product 4.
Owner:NINGXIA MEDICAL UNIV
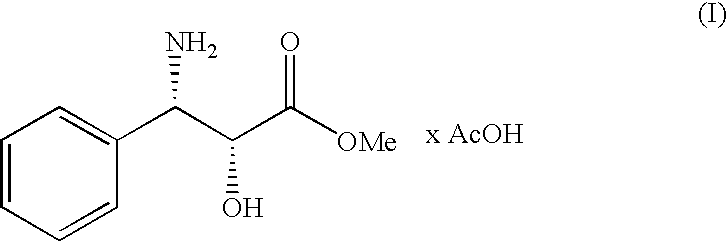
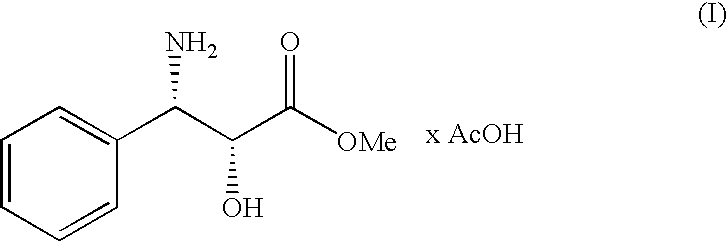
Process for the preparation of (2r,3s)-3-phenylisoserine methyl ester acetate salt
ActiveUS20100168460A1Organic compound preparationAmino-carboxyl compound preparationSerine methyl esterMedicinal chemistry
A process for the enantioselective preparation of (2R,3S)-3-phenylisoserine methyl ester acetate salt of formula (I) which is an useful building block for the synthesis of taxane derivatives. The process involves the resolution of racemic threo-phenylisoserine amide and its conversion into (I).
Owner:INDENA SPA
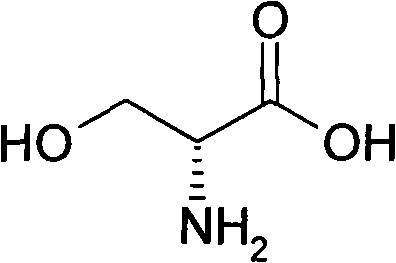
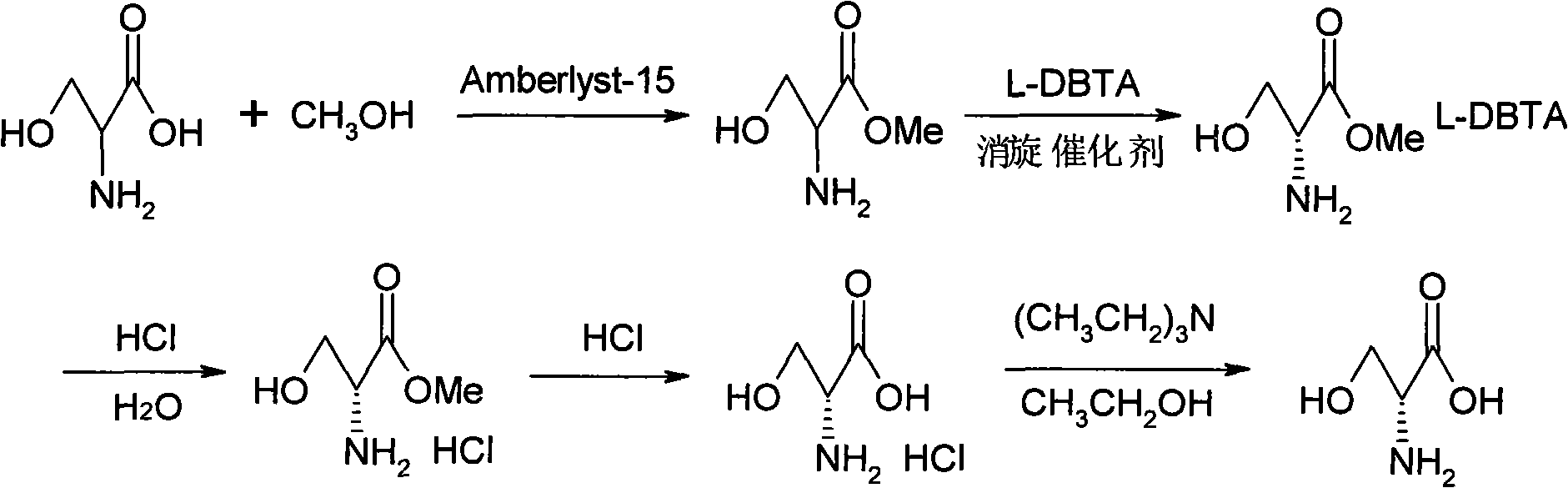
Method for preparing D-serine by kinetic resolution
ActiveCN101735085BHigh split yieldReduce operating costsOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterKinetic resolution
The invention discloses a method for preparing D-serine by kinetic resolution. The method takes DL-serine as raw material and is implemented by esterifying DL-serine with methanol under catalysis of Amberlyst-15 ion exchange resin to obtain DL-serinemethylester, carrying out dynamic kinetic resolution on DL-serinemethylester and resolving agent L-DBTA under action of racemization catalyst to obtain dibasic DL-serinemethylester.L-DBTA, dissociating with hydrochloric acid, and hydrolyzing to obtain D-serine, the target product of the invention. Compared with the prior art, by using the racemization catalyst in the invention, the dynamic continuous conversion of dibasic L-serinemethylester.L-DBTA into dibasic DL-serinemethylester.L-DBTA can be realized with the theoretic conversion rate being approximate to 100%, thus greatly enhancing yield of resolution, reducing operation cost, stabilizing product quality and being suitable for industrialized production.
Owner:SHANGHAI SHISI CHEM PROD
Synthesis method of methyl 2-acetylamino-3-chloropropionate
InactiveCN110642736ARelaxed reaction conditionsHigh reaction yieldOrganic compound preparationCarboxylic acid amides preparationSodium bicarbonateAcetic anhydride
The invention discloses a synthesis method of methyl 2-acetylamino-3-chloropropionate. The method comprises the following steps: adding L-serine methyl ester hydrochloride into dichloromethane, dropwise adding thionyl chloride A, carrying out a sectional controlled-temperature reaction, then performing cooling to 20 DEG C, adding water for layering, and removing impurities by activated carbon so as to obtain an aqueous 3-chloro-L-alanine methyl ester hydrochloride solution; controlling the temperature of the aqueous 3-chloro-L-alanine methyl ester hydrochloride solution to be 15-25 DEG C, dropwise adding an aqueous sodium bicarbonate solution and acetic anhydride at the same time, and keeping the temperature for 1-4 h after drop-by-drop addition is completed; and carrying out extracting, concentrating, recrystallizing, centrifuging and drying to obtain the methyl 2-acetylamino-3-chloropropionate. The system is heated in a sectional controlled-temperature mode, so the method has the advantages of mild reaction conditions, high reaction yield, and few byproducts; and the aqueous 3-chloro-L-alanine methyl ester hydrochloride solution is directly used as a reaction raw material, so that the yield and the purity of the final product are improved.
Owner:湖北宇阳药业有限公司
Novel hydrogel and preparing method thereof
ActiveCN110066315AImprove gelationPromote rapid formationPeptide preparation methodsEnd-groupSpecific immunity
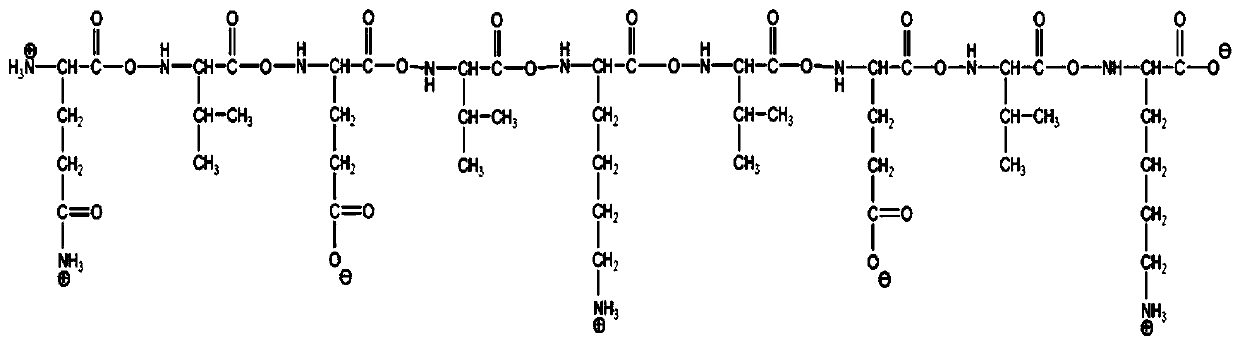
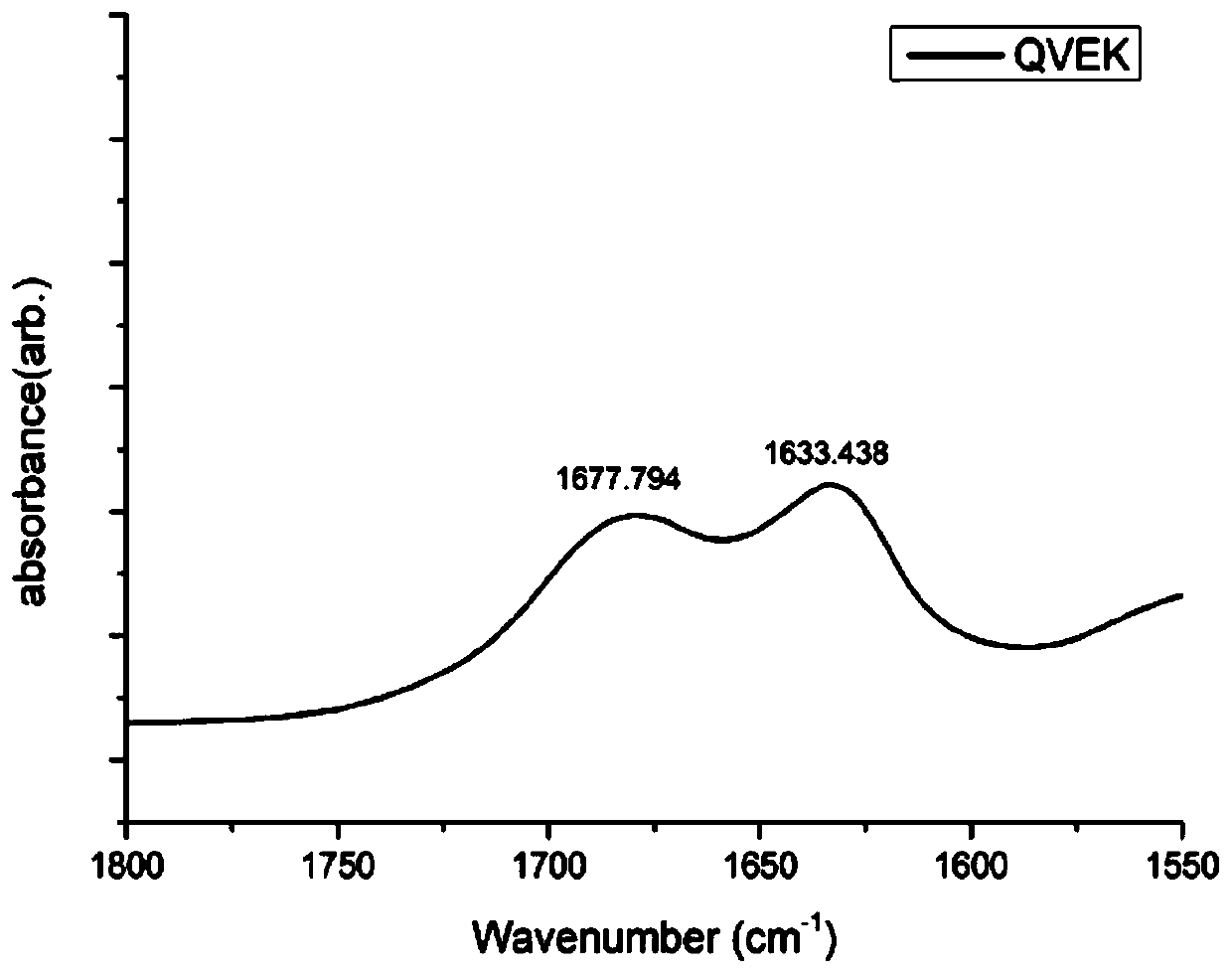
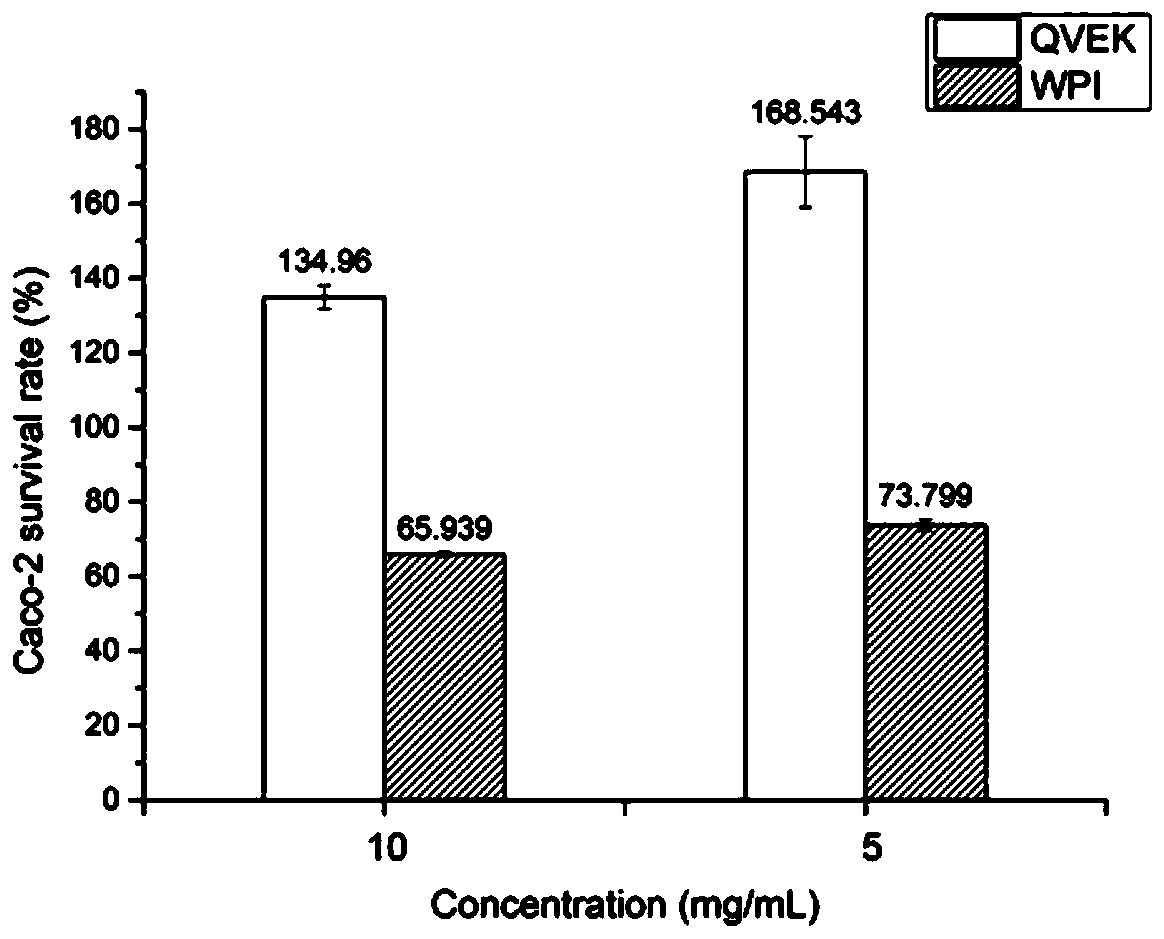
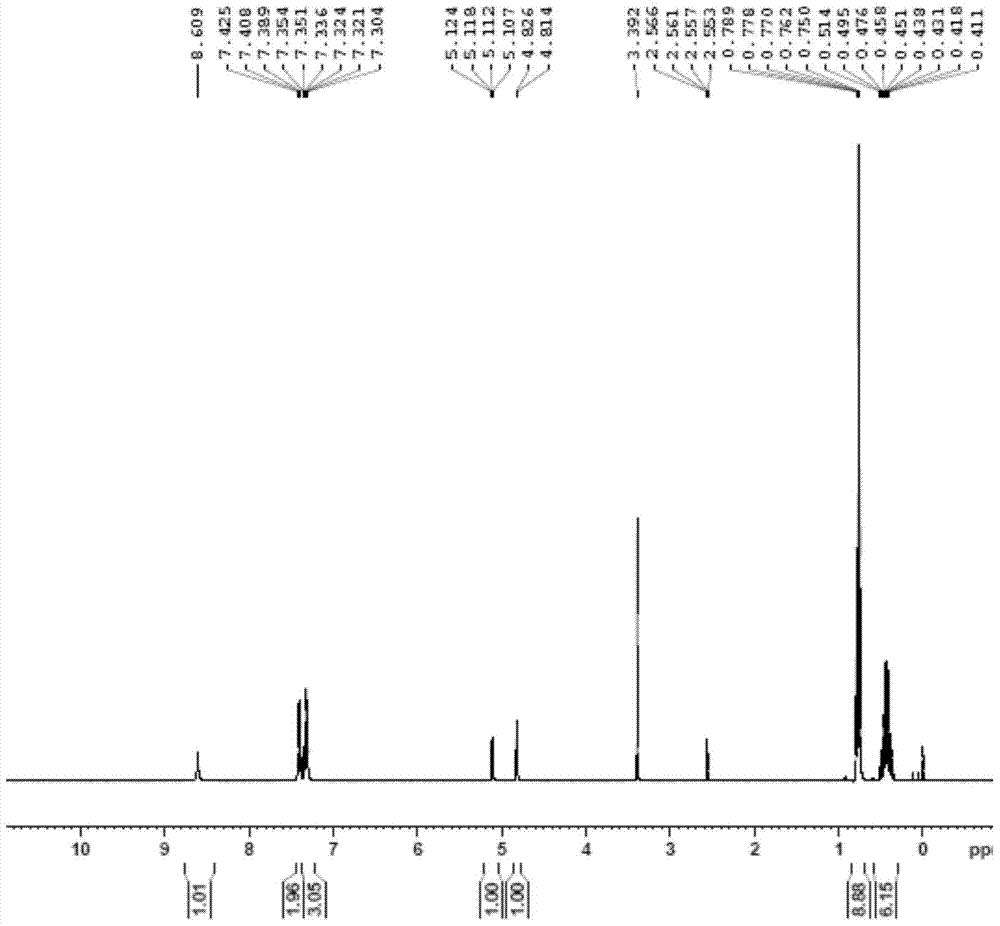
The invention discloses a novel hydrogel and a preparing method thereof, and relates to the technical field of gels. The novel hydrogel is prepared from synthetic peptide; the sequence of the synthetic peptide is glutamine-valine-glutamic acid-valine-lysine-valine-glutamic acid-valine-lysine, is easy to synthesize, and belongs to a complete amino acid sequence, and the end group of the sequence does not contain groups such as Nap-(naphthalene), Fmoc-(Fmoc-N-Me-Val-OH), Cbz-(n-carbobenzyloxy-L-serine methyl ester) and aliphatic chains; the easily-caused specific immunity can be effectively avoided or reduced, and the cell toxicity does not exist; the synthetic peptide has high gel property, and hydrogel can be rapidly formed through pH induction.
Owner:HUAZHONG AGRI UNIV
A kind of preparation method of chiral four-membered ring taxane side chain compound
ActiveCN104356157BLow costSimple and fast operationGroup 4/14 element organic compoundsSerine methyl esterSide chain
The invention relates to a preparation method of a chiral four-membered-ring taxane side chain compound and belongs to the field of pharmaceutical synthesis. The chiral four-membered-ring taxane side chain compound as shown in the formula I is prepared from a compound as shown in the formula II through a cyclization reaction. The method overcomes the defects of the relatively high cost of (2R,3S)-phenyl isoserine methyl ester in the prior art, and has the advantages of low cost of the used achiral material, simple equipment, easiness in operation, higher yield, and easiness in industrial production.
Owner:无锡紫杉药业股份有限公司
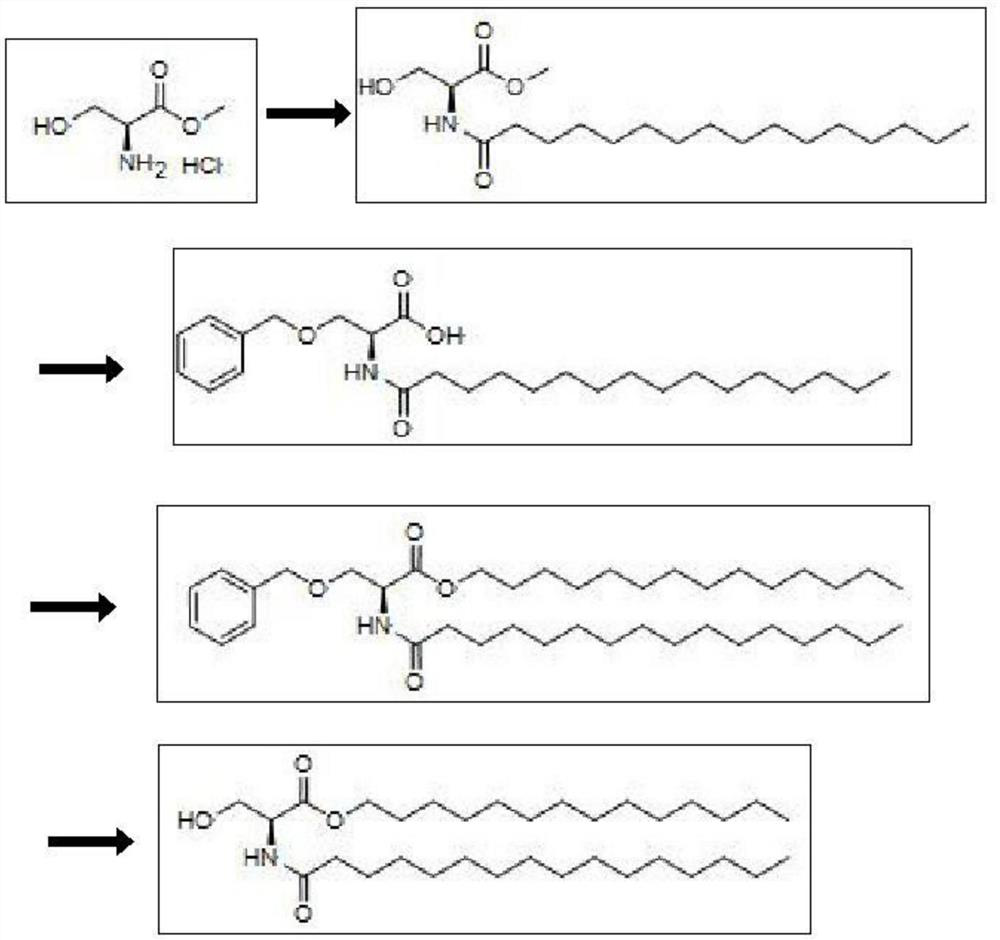
PMS synthesis method
ActiveCN112341354AImprove conversion rateHigh yieldOrganic compound preparationCarboxylic acid amides preparationPalmitoyl chlorideSerine methyl ester
The invention discloses a PMS synthesis method, and belongs to the technical field of organic synthesis. The method comprises the following steps: reacting L-serine methyl ester hydrochloride with palmitoyl chloride to obtain N-palmitoyl-L-serine methyl ester; carrying out benzyl protection on the N-palmitoyl-L-serine methyl ester, and hydrolyzing the methyl ester to obtain N-palmitoyl-O-benzyl-L-serine; esterifying the N-palmitoyl-O-benzyl-L-serine and tetradecyl alcohol to obtain N-palmitoyl-O-benzyl-L-serine tetradecyl ester; and finally, debenzylating the N-palmitoyl-O-benzyl-L-serine tetradecyl ester to obtain the PMS. According to the invention, cheap L-serine methyl ester hydrochloride is used as a raw material, and benzyl is used for protecting hydroxyl, so that the side reaction of esterification with tetradecyl alcohol when the hydroxyl in a serine parent is not protected is effectively avoided, the reaction conversion rate is improved, the difficulty of separation and purification caused by byproducts is reduced, and the method can be effectively suitable for industrial mass production.
Owner:HUBEI HUNTIDE BIOTECH
A kind of preparation method of 2,3-diaminopropionic acid methyl ester
ActiveCN109251150BStarting materials are readily availableMild reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterPhthalimide
The invention provides a method for preparing methyl 2,3-diaminopropionate, which uses serine as a raw material, uses thionyl chloride as a catalyst in methanol to prepare methyl serine, and then reacts with triphenylchloromethane to form Intermediate product 1, introduces Trt group, intermediate product 1 uses anhydrous tetrahydrofuran as solvent, under the catalysis of triphenylphosphine and DIAD, reacts with phthalimide to generate intermediate product 2, introduces Pht group, intermediate The Pht group of the product 2 was removed under the action of hydrazine hydrate to generate the intermediate product 3, and the Trt group of the final intermediate product 3 was removed in hydrochloric acid ethanol to obtain the final product 4.
Owner:NINGXIA MEDICAL UNIV
Preparation method of new taxane derivative
InactiveCN103254187AOvercome the disadvantage of many reaction stepsSolve the problem of isomeric impuritiesOrganic chemistryBulk chemical productionSerine methyl esterDocetaxel-PNP
The invention discloses a preparation method of new taxane derivative. The method provides an oxazoline side chain produced from reaction of (2R, 3S)-3-phenyl isoserine methyl esters and tribromoacetaldehyde, and then a new taxane derivative is coupled by 10-deacetyl baccatin III whose 7-poistion and 10-position are protected by trichloroacetyl group and oxazoline side chain. The preparation of docetaxel from taxane derivative which is used as an intermediate according to the invention has the advantages of simple synthesis step, few side reactions, high yield, non-isomer by-products, low impurity content, high yield, high purity, greatly reduced cost, and promotion of business preparation of docetaxel. The invention has important meanings in Chinese yew plant species and resource protection and tumour treatment cost reduction, etc., and has great economic benefits.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com